Stephen L. Foster, PharmD, FAPhA, FNAP, discussed the new vaccine and RSV prevalence at the National Community Pharmacists Association 2023 Convention and Expo.

Stephen L. Foster, PharmD, FAPhA, FNAP, discussed the new vaccine and RSV prevalence at the National Community Pharmacists Association 2023 Convention and Expo.
Research findings may support further COVID-19 vaccination in children.
Jean-Venable R. Goode, PharmD, director of the PGY1 Community Based Residency Program at Virginia Commonwealth University, discussed the new vaccine guidelines from ACIP.
Based on data from an ongoing clinical trial and other studies, the R21 vaccine has been shown to have a high degree of efficacy when administered just before the high transmission season, with data showing a 75% reduction in cases of malaria in the 12 months following the 3-dose series.
During a podcast hosted by Permanente Medicine, Sandra Fryhofer, MD, chair-elect of the Board of Trustees for the American Medical Association and a liaison to the CDC’s Advisory Committee on Immunization Practices, discussed the importance of vaccines for the fall and winter seasons.
Moderna plans to begin a phase 3 trial of the combination vaccine this year.
The monovalent vaccine offers a protein-based non-mRNA option.

Vaccines were tested against various strains of the SARS-CoV-2 virus.

By offering vaccines year-round instead of only during the typical flu season, community pharmacies can maximize profits and help patients live healthier lives.
Katalin Karikó, PhD, and Drew Weissman, MD, PhD, were cited "for their discoveries concerning nucleoside base modifications that enabled the development of effective mRNA vaccines against COVID-19."
Standout updates include a relaxed recommendation for patients with a history of egg allergies, and that all available influenza vaccines for the 2023 to 2023 flu season are quadrivalent.

The mRNA-1010 vaccine generated a powerful response against 4 influenza virus strains compared to traditional flu shots.
Patients who were vaccinated and reinfected showed symptoms of few lesions, little mucosal disease, and minimal analgesia requirements.
Vaxart’s VP1-based bivalent oral tablet vaccine was developed to target the norovirus GI.1 Norwalk and GII.4 Sydney strains, which are the predominant strains affecting humans.

Previous clinical data has shown that the vaccine also generates a strong response to the EG.5 and FL.1.5.1 variants.
According to the Centers for Disease Control and Prevention, tens of thousands of HPV-related cancer diagnoses were given per year from 2015 to 2019.
Study authors conclude that all children should be up to date with recommended COVID-19 vaccines and initiate the vaccination process immediately when products become available.
Resources with pertinent information are essential in motivating individuals to comply with vaccine recommendations.
The CDC and WHO are currently tracking several variants that are driving the majority of new cases around the world.

The respiratory syncytial virus vaccine (Abrysvo) is the first and only maternal RSV vaccine used to help protect infants from the virus.
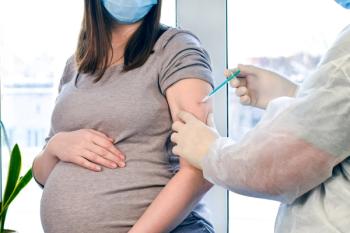
Receiving the vaccine during pregnancy is cited as crucial for both confronting disproportionate infant vulnerability and easing the disease burden for new and expecting mothers.

PXVX0317 has received Fast Track and Breakthrough Therapy designation from the FDA.
Moderna’s mRNA-1273 vaccine was associated with a lower risk of diagnosed COVID-19 when compared to Pfizer’s BNT162b2.
A Q&A with Marc Ost, co-owner of Eric’s RX Shoppe, which was one of the first community pharmacies to receive the new vaccine.