The company’s investigational ABCWY vaccine candidate will be reviewed by the FDA by February 14, 2025.

The company’s investigational ABCWY vaccine candidate will be reviewed by the FDA by February 14, 2025.
The use of tecovirimat for monkeypox and other non-variola orthopoxvirus infections is under an expanded access IND protocol, according to the CDC.
In a recent Morbidity and Mortality Weekly Report (MMWR), the results of a study showed 63% of those who died from HAV had at least 1 documented preexisting indication for vaccination.

Investigators saw there was a significant difference of lower treatment initiation in people younger than 30 years old, females, Hispanic and Asian individuals, and people who inject drugs.
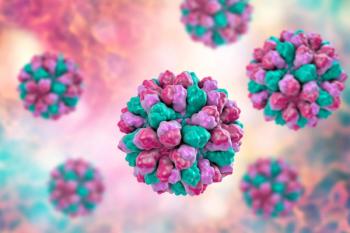
Vaxart’s VP1-based bivalent oral tablet vaccine was developed to target the norovirus GI.1 Norwalk and GII.4 Sydney strains, which are the predominant strains affecting humans.
The report references adult vaccines, in terms of volume of use, for influenza, diphtheria and tetanus (Td), diphtheria, tetanus and pertussis (TDaP), hepatitis B, herpes zoster and pneumococcal.
Pending authorization of the XBB.1.5 vaccine, the company said that doses would be available in time for fall vaccination.
In a large observational study, significant information was obtained about mRNA vaccine protection against acute infection and disease severity.

The antibiotic, marketed as Xacduro, is indicated for hospital-acquired bacterial pneumonia (HABP) and ventilator-associated bacterial pneumonia (VABP) caused by susceptible strains of bacteria called Acinetobacter baumannii.

Both Democrats and Republicans voted to bring back the act that attempts to improve use of antibiotics

The vaccine was 73.2% effective in children aged 6 months to 4 years when administered during the Omicron-dominant phase.
The updated vaccine schedule includes vaccinations for influenza, pneumococcal disease, measles, mumps, and rubella (MMR) and COVID-19.
The company is utilizing this platform to address new COVID-19 variants, combination vaccines, and potentially prevent HIV.