Dermatology
Latest News
Latest Videos
CME Content
More News

Pharmacists can help patients dodge sunburns and soak up summer fun with the best OTC sun care picks.

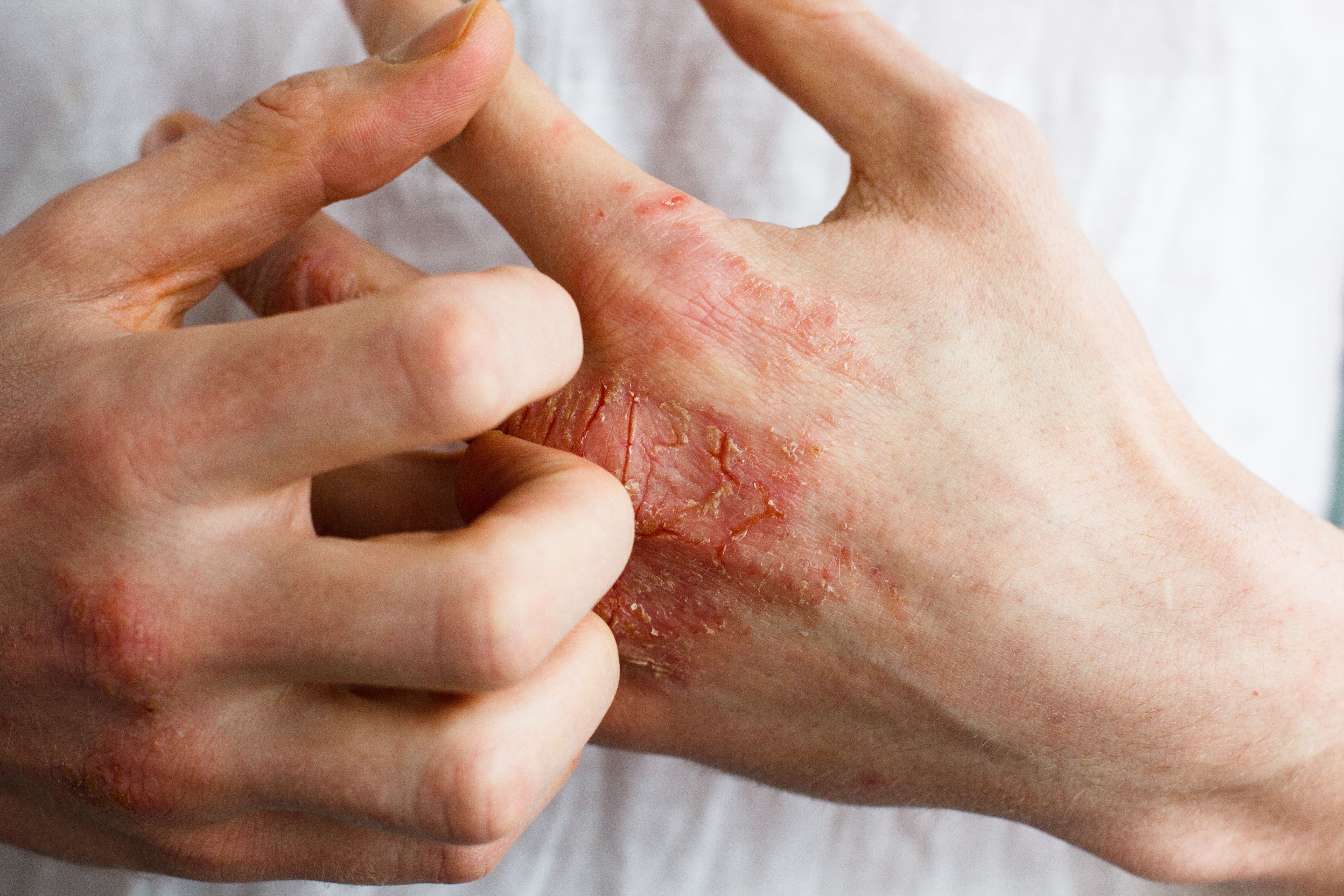
LEO Pharma’s topical medication was shown to be safe and effective in adult patients with moderate-to-severe hand eczema.

Researchers assessed the difference between hidradenitis suppurativa and other skin conditions regarding the initiating events, inflammatory signature, and molecular and clinical features of each disease.

Researchers explored the global disease burden of allergic-related skin diseases from 1990 to 2021 and projected future disease trends.

Researchers explored a variety of medicinal plants used for skin-related conditions and assessed their efficacy in treating UV-induced skin damage.
Researchers investigated the current treatment strategies for atopic dermatitis and how they related to ideas of corticophobia among providers.
Researchers reviewed current understandings of the role vaccines play in dermatology, what gaps persist, and what future research is necessary.

Bullous pemphigoid is a rare skin condition that causes large blisters filled with fluids that typically occur near skin creases, such as the upper thighs and armpits.

Researchers conducted a narrative review to explore the possibility of integrating social perspectives and aspects into dermatological care and research.
The review explored the “emerging” term gut-skin axis and aimed to decipher the bidirectional signaling of the skin and gastrointestinal tract during homeostatic and disease states.

PP405 targets the primary biological pathway that controls the natural hair growth cycle, separating it from existing therapies.

Researchers conducted a systematic review to summarize research that has addressed social media��’s impact on skin health promotion activities.

Deucravacitinib demonstrated a statistically significant inhibition of radiographic progression compared to placebo.

Using the “Psoriasis and Beyond” survey design, researchers addressed patients’ awareness of psoriatic diseases and their associated comorbidities.

Researchers aimed to understand the treatment goals and preferences of young patients with atopic dermatitis and their caregivers.
ASC40 blocks facial sebum production by inhibiting de novo lipogenesis in sebocytes and inflammation by decreasing cytokine secretion and Th17 differentiation.

Researchers explored the current evidence regarding women’s reactions to biological therapies for treating atopic dermatitis during pregnancy.

The supplemental new drug application for roflumilast (Zoryve) is approved for adult and pediatric patients 12 years and older.
Through a comprehensive review and meta-analysis, researchers identified the correlation between atopic dermatitis and dementia.

Researchers conduct a scoping review to update and determine what the best available options are for treating patients with skin-picking disorder.

In children aged 5 to 17 with skin disorders, researchers explore the risk of developing psychosocial comorbidities, sleep disturbances, and common related symptoms.

Researchers conducted a clinical review detailing the intersection of dermatology and suicide, explaining why skin conditions lead to increased risk of poor mental health.
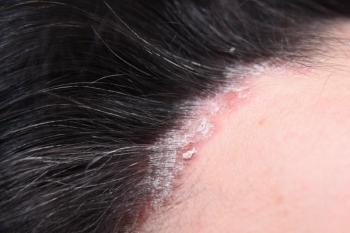
In a phase 3 study, 57% of patients treated with icotrokinra achieved an Investigator's Global Assessment score of 1 or 0 and a grade 2 or more improvement at week 16.

The therapy achieved positive results during a phase 2b trial on the primary endpoint of percent change in Eczema Area and Severity Index (EASI) score.
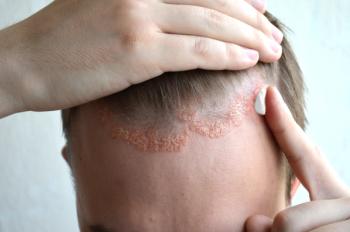
The therapy is currently under FDA review and has a PDUFA action date of May 22, 2025.