Biosimilars
Latest News
Latest Videos

CME Content
More News

The biosimilar is also approved for indications of Eylea, including neovascular age-related macular degeneration, macular edema following retinal vein occlusion, and diabetic retinopathy.

The FDA also grants the biosimilar provisional interchangeability designation, making the drug substitutable at the pharmacy level.

The FDA approves denosumab-nxxp (Bildyos and Bilprevda), enhancing access to vital bone health treatments for osteoporosis and cancer-related conditions.

This designation follows Hadlima’s low-concentration prefilled syringes and single-dose vials, which were designated in June 2024.

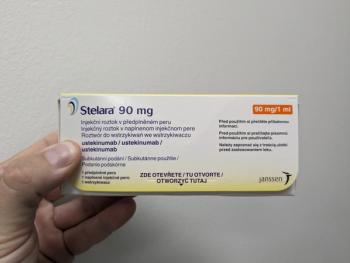
Ustekinumab is a medication used to treat and manage inflammatory conditions, including psoriasis, psoriatic arthritis, and inflammatory bowel disease.

Adalimumab-aaty (Yuflyma) prefilled syringes and autoinjectors are now fully interchangeable with the reference product adalimumab (Humira) across marketed dosage forms and strengths.
Pharmacists can substitute ustekinumab with the biosimilar at the pharmacy level, depending on their state laws.

Selarsdi is interchangeable for all indications of ustekinumab, including treatment of psoriatic arthritis, plaque psoriasis, Crohn disease, and ulcerative colitis.
Results are from an open-label extension of a phase 3 trial assessing the pharmacokinetics, efficacy, safety, and immunogenicity of adalimumab-aaty and its reference formulation Humira.

The FDA approved adalimumab-aaty in May 2023 for 8 indications, including rheumatoid arthritis, psoriatic arthritis, Crohn disease, ulcerative colitis, and plaque psoriasis.

Denosumab-bnht (Conexxence, Bomyntra) became the third biosimilar in 2025 to be approved for denosumab (Prolia, Xgeva).

Omalizumab-igec is approved to treat moderate to severe persistent asthma in patients 6 years and older whose symptoms are not well controlled with inhaled corticosteroids.

Denosumab-Bmwo is approved for all indications of the respective reference products, including osteoporosis and high-risk of fracture due to chemotherapy.
Yesintek from Biocon Biologics and Pyzchiva from Sandoz will have a patient assistance program that includes benefits verification and copay support.

Denosumab-dssb is approved as a 60 mg pre-filled syringe as a biosimilar for Prolia and 120 mg vial as a biosimilar for Xgeva.

The drug is the first rapid-acting insulin biosimilar product approved for use by the FDA, marking a significant milestone in diabetes treatment.

Chelsee Jensen, PharmD, BCPS, senior pharmacy specialist at the Mayo Clinic, discusses her career and how biosimilars will impact the pharmacy profession in the coming years.

A conversation with Chelsee Jensen, PharmD, BCPS, senior pharmacy specialist at the Mayo Clinic.

Drug Topics met with Amanuel Kehasse, PharmD, PhD, to discuss the barriers surrounding biosimilar implementation in the prescription drug market.
On the most recent episode of Over the Counter, Steve Callahan, Senior Director of Advisory & Insights at MMIT, talked with us about biosimilars and the Inflation Reduction Act.
Steve Callahan, Senior Director of Advisory & Insights at MMIT, discussed out-of-pocket costs and the impact biosimilars are predicted to have for patients in the future.
Steve Callahan, Senior Director of Advisory & Insights at MMIT, sat down with Drug Topics to discuss industry trends behind biosimilars.
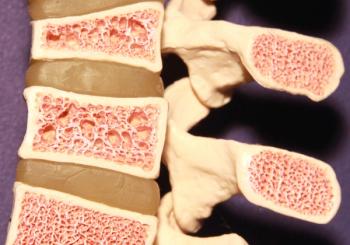
The cost effectiveness of biosimilar denosumab for postmenopausal osteoporosis varies significantly based on factors like adherence, subsequent therapies, and treatment duration.

The approval is supported by results from phase 3 multi-regional clinical trials conducted in patients with plaque psoriasis.

The approval of eculizumab-aagh offers more treatment options for patients with rare diseases PNH and aHUS.