
Compounding
Latest News
Advertisement
Latest Videos
CME Content
Advertisement
More News
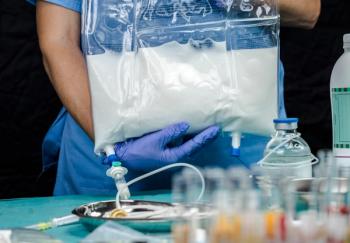
Compounding parenteral nutrition solutions is a complex process with a high risk for error, which can lead to serious adverse outcomes.

A study found that the average difference between the pilocarpine HCL solution and Pilogel discs was -3.25 mmol/L and 0.69 mmol/L.
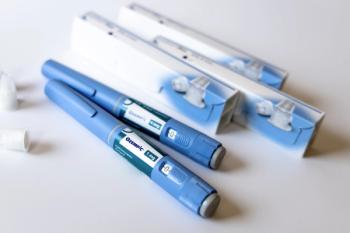
Novo Nordisk is the only company with FDA-approved products containing semaglutide.

The long-awaited revised USP <797> guidelines will become official on November 1, 2023.

The FDA has updated its compounding guidelines for licensed pharmacies, hospitals, and health systems.
Advertisement
Advertisement
Trending on Drug Topics
1
TrumpRx Officially Launches, Introduces Drug Prices
2
As Immunization Schedules Change, Vaccine Confidence Among Patients Falters
3
President Trump Signs Sweeping, Unprecedented Federal PBM Reform
4
Q&A: PBMs, Community Pharmacy’s Next Move Following PBM Reform Signing
5



























