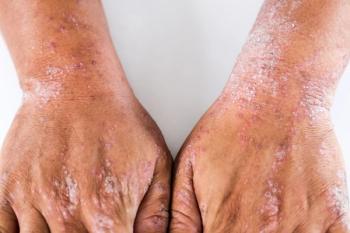
A lack of diversity in clinical trials has hampered health care providers from understanding the nuances of diseases across specific patient populations.

A lack of diversity in clinical trials has hampered health care providers from understanding the nuances of diseases across specific patient populations.

Medical spending on pediatric mental health conditions accounted for 46.6% of all pediatric medical spending in 2021.

Manufacturer ARS Pharma will submit the data as part of a response to a CRL received in 2023.
The FDA states the revision to the emergency use authorization (EUA) is the next phase in the transition from the use of EUA-labeled nirmatrelvir and ritonavir to the use of NDA-labeled nirmatrelvir and ritonavir.
Study results found that maternal COVID-19 vaccination was safe from the perspective of infant neurodevelopment up to 18 months of age, addressing concerns about in utero vaccine exposure.

These findings can help health care professionals develop a family-centered treatment plan for pediatric type 1 diabetes, considered a “family disease” by study authors.


An FDA-approval of Omalizumab would help reduce allergic reactions among 17 million people in the United States who have confirmed food allergies.

According to a poster abstract presented at the ASHP Midyear Clinical Meeting & Exhibition, specialty pharmacy services were used for a variety of conditions that have been reported in adult populations.

According to a poster presented at ASHP Midyear 2023, stress ulcer prophylaxis (SUP) did not increase adverse effects, suggesting limited preventive benefits for gastrointestinal bleeding (GIB) in neonates.

Levetiracetam and clobazam can cause Drug Reaction with Eosinophilia and Systemic Symptoms, which can start as a rash but progress quickly, potentially resulting in injury to internal organs, hospitalization, and death.

This season marks the fourth to be classified as high severity since the 2009 influenza A(H1N1) pandemic.

ADP101 is designed to treat allergy to 1 or more of the world’s most significant allergens including cashew, almond, chicken’s egg, cow’s milk, codfish, peanut, pecan, pistachio, salmon, sesame, soy, shrimp, walnut and wheat.

Because of misclassification of diabetes type and the absence of distinction between risk factors, such as birth weight, and diabetes type, uncertainty exists regarding the relationship between obesity and T1D.
The FDA is advising health care professionals who administer the vaccine to make sure the correct volume of the vaccine is withdrawn from the vial and the correct dose is administered to the patient.
A poster session at the 2023 American Academy of Pediatrics National Conference & Exhibition, featured a complex pediatric case involving SARS-CoV-2, bacterial coinfection, multiorgan failure, thrombotic microangiopathy, and systemic hyperinflammation.

A new study in the New England Journal of Medicine found no significant difference in the number of children who received a T1D diagnosis between those with and without a SARS-CoV-2 infection.
Standout updates include a relaxed recommendation for patients with a history of egg allergies, and that all available influenza vaccines for the 2023 to 2023 flu season are quadrivalent.

In addition to approving these generics for children, adolescents and adults with ADHD, the generic chewable tablets and capsules are also approved for adults with moderate to severe binge-eating disorder.
Study authors conclude that all children should be up to date with recommended COVID-19 vaccines and initiate the vaccination process immediately when products become available.

PXVX0317 has received Fast Track and Breakthrough Therapy designation from the FDA.

A 510(k) clearance, or a Premarket Notification, requires device manufacturers to notify the FDA at least 90 days ahead of registration with their intent to market a medical device.

A study from Bavaria, Germany, found the frequency was higher among boys and children younger than the median age of 6.5 years during the pandemic.
Investigators said that RSV-prevention products and socioeconomic determinants of health for AI/AN children are “urgently needed.”
The approval follows a unanimous vote of support from the FDA Antimicrobial Drugs Advisory Committee.

The acceptance comes 5 days after the agency approved delandistrogene moxeparvovec-rokl to treat ambulatory pediatric DMD patients aged 4 to 5 years.

The accelerated approval of delandistrogene moxeparvovec-rokl is “based on expression of Elevidys micro-dystrophin observed in patients treated with ELEVIDYS,” according to Sarepta Therapeutics.

According to the FDA, the approvals “provide a new class of medicines taken by mouth to treat pediatric T2D.”

Odevixibat can now be used to treat cholestatic pruritus in young patients with Alagille syndrome (ALGS).

Published: May 15th 2023 | Updated:
Published: April 7th 2023 | Updated:

Published: March 22nd 2023 | Updated:

Published: March 28th 2023 | Updated:

Published: December 5th 2023 | Updated: