A recent poll showed that some parents do not discuss vaccines with their child’s regular doctor, with many choosing to not have their child receive any vaccines.

A recent poll showed that some parents do not discuss vaccines with their child’s regular doctor, with many choosing to not have their child receive any vaccines.

The COVID-19 pandemic disrupted routine immunization programs around the world.
Confidence in vaccines is lower post-pandemic across all demographic groups
Communication and education are critical to patient confidence in emergency use authorization vaccines.

Mitchel Rothholz, RPh, MBA, and Jeff Goad, PharmD, MPH, share thoughts on COVID-19 vaccines and boosters in anticipation of an uptick in cases in the coming months.

Key opinion leaders share insights regarding considerations for COVID-19 boosters amid variants.
Addressing hesitancy is more than just offering pertinent information.

In a recent study, researchers found that text message reminders led to increased influenza vaccination rates, along with many receiving the vaccine sooner.
Human papillomavirus vaccines can help prevent cancer, but uptake still lags.
Phase I/II data indicate that new investigative pneumococcal conjugate vaccine shows efficacy in preventing disease in adults.

Offering immunization services at the pharmacy promotes public health and strengthens patient-pharmacy staff relationships.
Results of multiple studies were presented at IDWeek 2022.

After multiple approvals and emergency use authorizations, It’s time to take stock of the state of pediatric COVID-19 vaccines.
Adult vaccine uptake lags and understanding the reasons could be important.
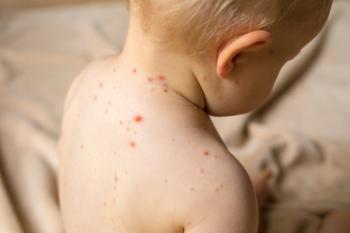
The US was the first country to begin universal vaccination of children against varicella.
As vaccine hesitancy grows, fewer children are receiving routine childhood vaccines.
A selection of the latest COVID-19 vaccine research out of IDWeek 2022.

What does the uptake look like for one of the most common vaccines given during pregnancy—and what does this mean for the future?
Adults who receive influenza and pneumococcal vaccines may be more likely to receive COVID-19 vaccines and boosters.

Pharmacists can play a key role in helping to improve backsliding vaccination rates.
Final thoughts are provided as the panelists consider opportunities in the field of influenza vaccination.

The panel provides opinions on the incorporation of vaccine awareness and administration from public and state levels.

Distinctions between vaccine types are explored, as well as considerations such as egg-based allergies.

Vaccination discussion continues with a focus on navigating patient concerns and potential adverse effects to vaccine administration.

Infants of fully vaccinated mothers had fewer cases of hospitalization from COVID-19 than those unvaccinated.