Fragmin, a low-molecular-weight heparin dalteparin sodium injection, was approved for symptomatic venous thromboembolism.

Fragmin, a low-molecular-weight heparin dalteparin sodium injection, was approved for symptomatic venous thromboembolism.
Venetoclax, a BCL-2 inhibitor first approved in 2016, is now indicated for chronic lymphocytic leukemia and small lymphocytic lymphoma.
Approval marks the first anti-PD-L1 therapy combination for RCC.
Ruzurgi approval for rare autoimmune disorder may be an olive branch.
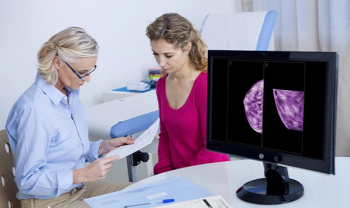
Recent trial shows greater invasive disease-free survival when treated with Kadcyla , (ado-trastuzumab emtansine)
New drug combines dapagliflozin, saxagliptin, and metformin.
Two different versions of tafamadis get the FDA’s nod to treat a rare heart disease.
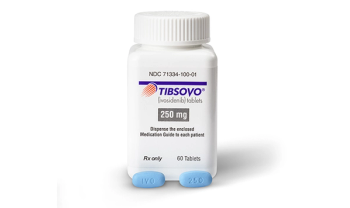
Tibsovo (ivosidenib) is an isocitrate dehydrogenase1- inhibitor, originally approved in 2018.
First vaccine approved for all four Dengue virus serotypes in children and teens ages 9 through 16, but comes with major restrictions and controversy.

Results from a recent ELLIPSE trial indicate superiority in reducing blood sugar when compared to the placebo.

Benlysta is the first approved treatment for children with lupus.
Eticovo (etanercept-ykro) is a TNF blocker, biosimilar to Enbrel (etanercept).

New lotion from Ortho Dermatologics expected June 2019.
DUAKLIR PRESSAIR (aclidinium bromide and formoterol fumarate) provides LAMA/LABA therapy.
Diacomit (stiripentol) is indicated for rare, severe form of pediatric epilepsy.
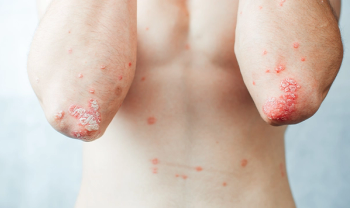
Indicated for adults with moderate to severe plaque psoriasis.

Esketamine comes with close supervision requirements. 800 clinics have already been approved and dispensed initial applications.

FDA granted final approval to drug originally tentatively approved in 2018.
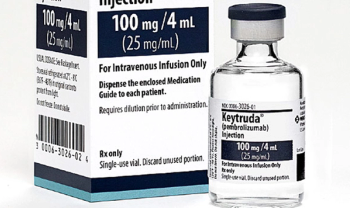
The FDA has recently approved new dosage form and two new indications for the drug.
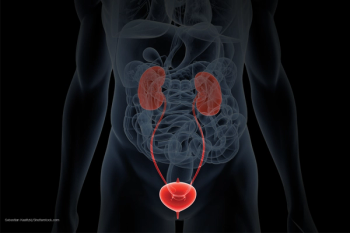
Erdafitinib is first targeted therapy for FGFR3 or FGFR2 genetic alteration.

Safety alert issued requiring warning about concurrent alcohol use.
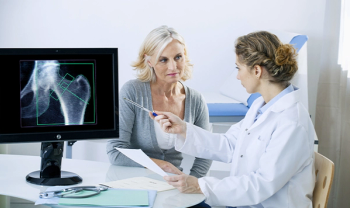
Evenity (romosozumab-aqqg) has been approved to treat postmenopausal women at high risk of bone fractures.

Women no longer require nine months of contraception post cessation of the drug.
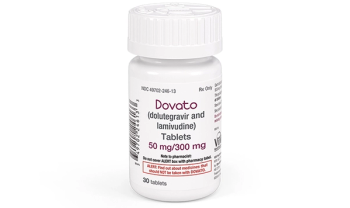
First-ever dual-drug, single tablet for HIV offers lighter treatment regimen.

Solriamfetol is approved for adult patients suffering from the effects of narcolepsy and OSA.
The next-generation oral drug is the first to be approved specifically for secondary progressive multiple sclerosis in more than 15 years.

First drug approved for PPD use to remain under REMS program.
Ongoing valsartan shortages due to recalls prompt FDA prioritization.

Indicated for breast cancer, and gastric or gastroesophageal junction adenocarcinoma.