
The FDA granted priority review for this drug due to the longstanding need for a treatment strategy that could improve upon the standard of care.
Kathryn Wheeler, PharmD, BCPS, is assistant clinical professor of pharmacy practice, University of Connecticut School of Pharmacy, Storrs, Conn.

The FDA granted priority review for this drug due to the longstanding need for a treatment strategy that could improve upon the standard of care.
This novel mechanism of action provides an alternative therapy for patients with recurrence or those who may not tolerate current first-line therapies.

The FDA approved imetelstat (Rytelo) in June 2024.

Cefepime/enmetazobactam was granted a 5-year exclusivity extension under GAIN Act legislation.

Berdazimer topical gel is the first in its class and is the only topical prescription medication to treat molluscum contagiosium that can be applied by patients or caregivers outside of a medical setting.

Last August, zuranolone (Zurzuvae) became the first FDA-approved pill to treat postpartum depression in adults.

Fezolinetant (Veozah) is approved for the treatment of moderate to severe vasomotor symptoms (ie, hot flashes and night sweats) related to menopause.

Daprodustat increases erythropoietin production, leading to an increase in the production of red blood cells.

Tirzepatide is a once-weekly subcutaneous injection to treat type 2 diabetes in conjunction with diet and exercise.
Korsuva is approved for treating moderate to severe pruritis associated with chronickidneydisease
On June 2,2021, ibrexafungerp (Brexafemme; Scynexis, Inc) was approved by the FDA for vulvovaginal candidiasis (VVC) in adult women and girls who have begun menstruating.

Viloxazine is a selective norepinephrine reuptake inhibitor and is the first new nonstimulant medication for treatment of ADHD in children in more than 10 years.


On February 27, 2020, the FDA approved an intravenous solution of amisulpride (Barhemsys; Acacia Pharma) to treat and prevent postoperative nausea and vomiting.

The FDA has approved lumateperone tosylate (Caplyta™, Intra-Cellular Therapies) for treatment of schizophrenia in adults.
On December 23, 2019, the FDA approved ubrogepant for the acute treatment of migraine with and without aura in adult patients.
The first drug approved to specifically treat post-partum depression.
What you need to know about the serotonin-4 receptor agonist approved for adult patients.
Sublingual form approved for short-term use.

The latest New Drug Review covers Amgen’s migraine medication.

Our latest New Drug Review covers Dova’s most recent medication.
What pharmacists need to know about the new antibody approved for multi-drug-resistant HIV-1.
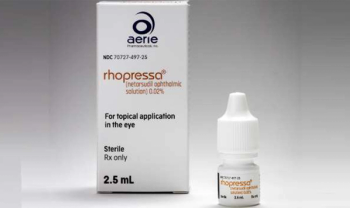
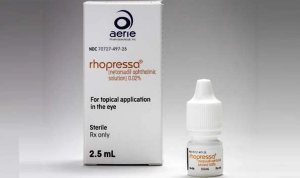
Netarsudil opthalmis solution (Rhopressa, Aerie Pharmaceuticals), is approved by the FDA for treatment of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension.
Published: July 14th 2021 | Updated:

Published: June 22nd 2020 | Updated:

Published: July 12th 2022 | Updated:
Published: March 2nd 2018 | Updated:
Published: May 2nd 2018 | Updated:

Published: August 1st 2018 | Updated: