
TNF blocker biosimilar to Humira (adalimumab, Abbvie).

CD20-directed antibody indicated for several cancers and other conditions.

Distributed by Invagen, a subsidiary of Cipla Ltd.
Fourth indication for the drug since 2014.

Combination drug indicated for infections caused by susceptible gram-negative bacteria.
First oral drug for advanced or metastatic bladder cancer.
Fourth approval for use in assessing myocardial perfusion and late gadolinium enhancement.

COMT inhibitor to be used as an adjunctive treatment to levodopa/carbidopa.
Nuclear export inhibitor indicated in combination with dexamethasone.
Now approved to be used in combiantion with lenalidomide and dexamethasone.
Complement inhibitor is the first treatment for the rare autoimmune disorder.
First treatment approved for the condition with nasal polyps.
New features to ease injection administration.
The first drug approved to specifically treat post-partum depression.
Approved to treat pediatric patients ages 6 years and older with cystic fibrosis
First non-insulin drug for pediatric use approved in nearly 20 years.
Indications include breast cancer, metastatic gastric cancer, and metastatic gastroesophageal junction adenocarcinoma.

Both responder and remitter patients experienced a statistically significant longer time to relapse with esketamine compared to the placebo
Drug targets the CD79b protein on B-cells.
Zerbaxa is a cephalosporin antibacterial and beta-lactamase inhibitor combination drug originally approved for abdominal and urinary tract infections.

Patients on Emgality experienced an average of 8.7 fewer weekly episodic cluster headaches.
This thalidomide analogue is also indicated for multiple myeloma and myelodysplastic syndromes.
New indication adds to the oral antipsychotic’s portfolio of conditions.
First PIK3CA inhibitor treatment approved, along with diagnostic test.
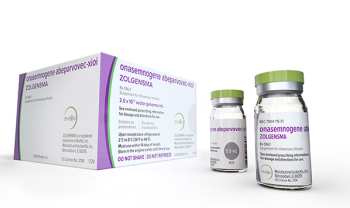
Zolgensma (onasemnogene abeparvovec-xioi) is an adeno-associated virus vector-based gene therapy
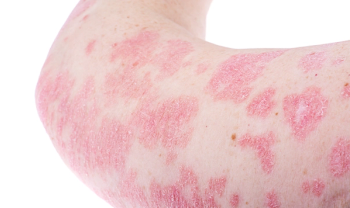
Vitamin D analog foam indicated for topical treatment of plaque psoriasis in teens.

Oral solution now approved for adults and pediatric patients.
Update: now available from three manufacturers.

Nayzilam is a benzodiazepine antiepileptic nasal spray.