Fourth indication expansion for the 2009 drug.

Reduces risk for heart failure and cardiovascular disease hospitalizations.

Drug able to treat 18,000 people will cost over $300k per year.
Available for prescribing starting January 2020
First approved in 2018 for patients 12 years of age and older.
Originally approved in 1995 for use in adults only.

Administered via a once-daily transdermal patch.
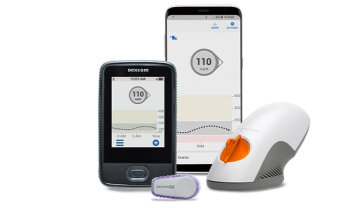
New CGM eliminates fingersticks, introduces new data presentation.

Afamelanotide released through subcutaneous implant.
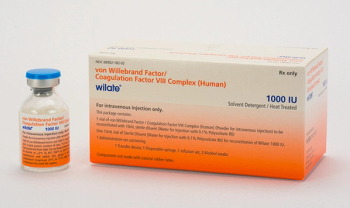
Also indciated to control bleeding and perioperative management.

First new treatment of its kind in over 20 years.

For granulomatosis with microscopic polyangiitis in children.
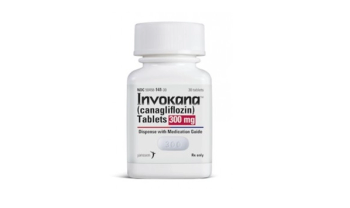
Now approved for risk reductions in patients with type 2 diabetes mellitus.
Will be part of the U.S.’s public health emergency medical supplies and pharmaceuticals.
Monoclonal antibody for autoimmune and neurodegenerative disorders.
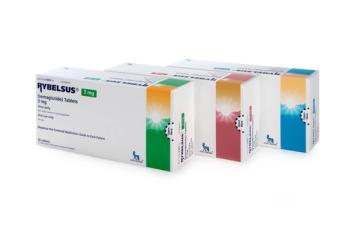
Now approved in 7mg and 14 mg tablets.
Derived from cultured adult bone marrow-derived mesenchymal stem cells that have undergone temporary genetic modification.
Monoclonal antibody shows potential for lupus nephritis treatment.
Combination therapy for advance endometrial carcinoma.
NHE3 inhibitor administered as 50 mg oral tablets.
Investigational oral MET kinase inhibitor from Merck.

Oral supplements for metabolic disorder.
First generic specialty injections approved for Novadoz Pharmaceuticals.
Cariprazine demonstrated greater improvement on the MADRS.

New pleuromutilin antibiotic IV and oral tablets.

Humira successor indicated for adults with inadequate response or intolerance to methotrexate.

First new drug approval for the condition in nearly a decade.

Kinase inhibitor oral capsules.
Black box warns of increased risk of blood clots and death.