
See how some students have opted for a unique conclusion to their studies.

See how some students have opted for a unique conclusion to their studies.
20% subcutaneous immunoglobulin therapy.

Two new best practices, a new classification, and 5 updated existing rules.

Takeaways from a roundtable at ASHP 2019.

Presented at the ASHP Midyear 2019.
Based upon results from a military population study presented at ASHP 2019.

The pair were recognized for contributions to the association and industry.

Results at ASHP 2019 suggest potential for administration errors without proper guidance.

Biosimilar to Remicade

Allergan’s positive phase 3 trial results indicate a potential use for the CGRP receptor agonist.
Monoclonal antibody originally approved in 2016.
Recent approval under new international Project Orbis.
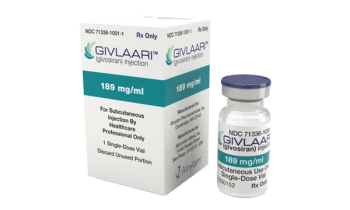
Ultra-rare, potentially life-threatening genetic disease.
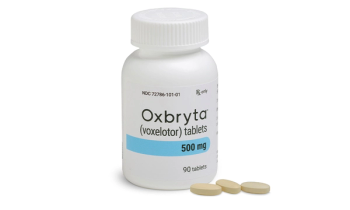
Hemoglobin S Polymerization inhibitor oral treatment.
Approved for patients 18 years of age and older
Antibiotic with a novel penetrative mechanism.

Expected U.S. launch in 2023.
First and currently only biological targeting P-selectin.
MCL is an aggressive form of NHL

Shown to reduce regular RBC transfusions in adults with beta thalassemia.
Fourth filgrastim biosimilar from Sandoz, expected ASAP.

Could lead to falsely high or low results, especially troponin assays.
Approved for ages 65 and older.
First and currently only rifabutin-based H. pylori therapy approved.
Delayed-release oral capsules for various forms of the disease.
Commercial launch suspended due to manufacturer liquidity.
New formulation improves safety for patients.
Previously approved for IV infusion.
Fourth indication expansion for the 2009 drug.

Reduces risk for heart failure and cardiovascular disease hospitalizations.

Published: November 19th 2018 | Updated:

Published: December 6th 2018 | Updated:

Published: December 17th 2018 | Updated:

Published: December 28th 2018 | Updated:
Published: January 16th 2019 | Updated:

Published: February 5th 2019 | Updated: