
The agreement will bring real-world data to cancer treatment development.

The agreement will bring real-world data to cancer treatment development.

Earn up to 19.25 hours of CE.
This thalidomide analogue is also indicated for multiple myeloma and myelodysplastic syndromes.
New indication adds to the oral antipsychotic’s portfolio of conditions.
First PIK3CA inhibitor treatment approved, along with diagnostic test.
Zolgensma (onasemnogene abeparvovec-xioi) is an adeno-associated virus vector-based gene therapy
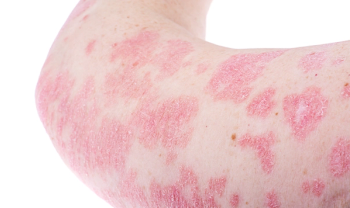
Vitamin D analog foam indicated for topical treatment of plaque psoriasis in teens.

Oral solution now approved for adults and pediatric patients.
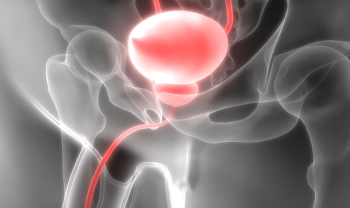
Update: now available from three manufacturers.

Nayzilam is a benzodiazepine antiepileptic nasal spray.
Fragmin, a low-molecular-weight heparin dalteparin sodium injection, was approved for symptomatic venous thromboembolism.
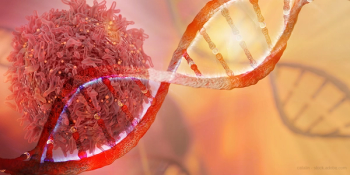
Venetoclax, a BCL-2 inhibitor first approved in 2016, is now indicated for chronic lymphocytic leukemia and small lymphocytic lymphoma.

Xellia Pharmaceuticals will manufacture essential antibiotics for Civica Rx.
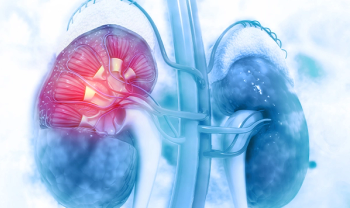
Approval marks the first anti-PD-L1 therapy combination for RCC.
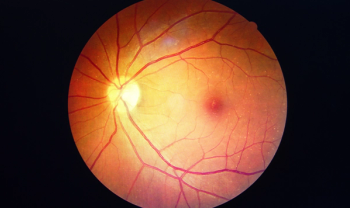
Originally approved in 2011, aflibercept is a vascular endothelial growth factor (VEGF) inhibitor.
New indication joins a series of single-agent and combination treatments for various cancers.

Finalized rule is a step toward increasing drug pricing transparency.
Ruzurgi approval for rare autoimmune disorder may be an olive branch.

Nearly 12 million pounds of medications collected since the initiative’s inception.
Recent trial shows greater invasive disease-free survival when treated with Kadcyla , (ado-trastuzumab emtansine)
New drug combines dapagliflozin, saxagliptin, and metformin.

New services will start with select pharmacists before expanding to Walgreens team memebers and potentially a nationwide resource.
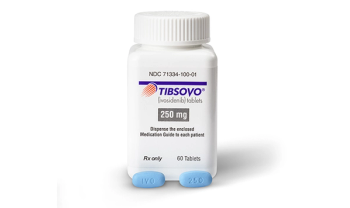
Tibsovo (ivosidenib) is an isocitrate dehydrogenase1- inhibitor, originally approved in 2018.
First vaccine approved for all four Dengue virus serotypes in children and teens ages 9 through 16, but comes with major restrictions and controversy.


Results from a recent ELLIPSE trial indicate superiority in reducing blood sugar when compared to the placebo.

Benlysta is the first approved treatment for children with lupus.
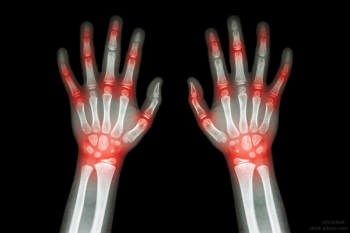
Eticovo (etanercept-ykro) is a TNF blocker, biosimilar to Enbrel (etanercept).

The campaign places emphasis on Americans' role in proper removal and disposal of opioids from their home.

New lotion from Ortho Dermatologics expected June 2019.