
Drug able to treat 18,000 people will cost over $300k per year.

Drug able to treat 18,000 people will cost over $300k per year.
Available for prescribing starting January 2020
First approved in 2018 for patients 12 years of age and older.
Originally approved in 1995 for use in adults only.

Administered via a once-daily transdermal patch.
Noteworthy diabetes management products and updates making waves this year.

Consumers should stop use and report any adverse events.
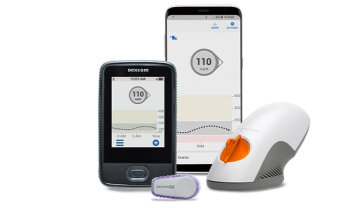
New CGM eliminates fingersticks, introduces new data presentation.

Afamelanotide released through subcutaneous implant.
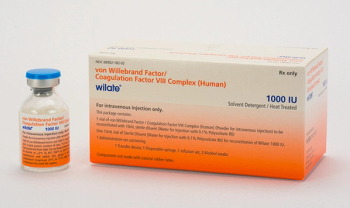
Also indciated to control bleeding and perioperative management.
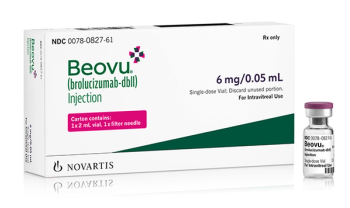
Injection for leading cause of severe vision loss and legal blindness.

First new treatment of its kind in over 20 years.

Currently only approved for men and transgender women; future trials expected.

For granulomatosis with microscopic polyangiitis in children.
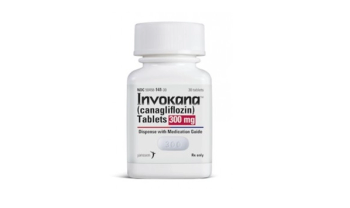
Now approved for risk reductions in patients with type 2 diabetes mellitus.
Monoclonal antibody for autoimmune and neurodegenerative disorders.
Now approved in 7mg and 14 mg tablets.
Derived from cultured adult bone marrow-derived mesenchymal stem cells that have undergone temporary genetic modification.
Monoclonal antibody shows potential for lupus nephritis treatment.
Combination therapy for advance endometrial carcinoma.
NHE3 inhibitor administered as 50 mg oral tablets.

Patients are still allowed to take medicines as prescribed.
Investigational oral MET kinase inhibitor from Merck.

Oral supplements for metabolic disorder.
Oral kinase inhibitor for SSc-ILD.
First generic specialty injections approved for Novadoz Pharmaceuticals.
Though decreasing, initiatives and awareness will generate more revenue.
Rare instances of worsening liver function reported to FDA.

Grant-funded program to bring more opportunities for safe disposal.
