
CDC updates vaccination guidelines, emphasizing individual choice for COVID-19 and standalone chickenpox shots, while addressing vaccine hesitancy and misinformation.
Ashley Gallagher is an assistant managing editor at Drug Topics®. She graduated in 2020 in journalism and mass communications and received her master’s degree in 2025 in digital journalism from St. Bonaventure University. Previously, she worked as a pharmacy technician for a retail chain.

CDC updates vaccination guidelines, emphasizing individual choice for COVID-19 and standalone chickenpox shots, while addressing vaccine hesitancy and misinformation.

TrumpRx drives Most-Favored-Nation drug pricing, cutting US costs for glucagon-like peptide-1, insulin, and fertility medications.

Pharmacists push for provider status and PBM overhaul as IRA policies squeeze pharmacies—what it means for access to vaccines, PrEP, and vital meds.
Pfizer's phase 2b study reveals promising results for its monthly GLP-1 therapy, enhancing weight loss and medication adherence in obesity management.

Novo Nordisk's CagriSema shows significant improvements in blood glucose control and weight loss for type 2 diabetes patients in recent trial results.

Inhaled Technosphere insulin revolutionizes diabetes management for children and pregnant women, addressing treatment adherence and psychological barriers effectively.

A plant-based lifestyle intervention can lead to diabetes remission and weight loss, transforming traditional diabetes management strategies.

High-dose vitamin D3 shows promise in improving metabolic health for type 2 diabetes patients, enhancing glycemic control alongside traditional treatments.

A recent survey reveals that over half of US patients face challenges with prescription costs, highlighting the need for better cost transparency and support.

Pharmacists enhance travel medicine by offering tailored consultations, risk assessments, and vaccine management, ensuring safe international travel for patients.

Consultant pharmacists enhance vaccination efforts in long-term care, addressing challenges like vaccine fatigue while advocating for age-friendly care and improved patient outcomes.

The Pharmacy Quality Alliance enhances patient outcomes by addressing social determinants of health through effective community partnerships and pharmacist involvement.

PQA releases the SDOH Resource Companion Guide, highlighting successful partnerships and strategies to address health-related social needs effectively.

Consultant pharmacists enhance long-term care by integrating vaccination strategies, educating staff, and addressing the unique needs of older adults.

Lumateperone shows promising remission rates for major depressive disorder, offering hope for sustained relief in adults when combined with antidepressants.

Fremanezumab significantly reduces migraine days in children and adolescents, offering a vital preventive option for managing episodic migraines.

Gestational diabetes significantly impacts postpartum cardiovascular health, especially in women over 35, highlighting the need for timely interventions.

Educational interventions and pharmacist engagement enhance patient safety in OTC medication use, addressing knowledge gaps and promoting responsible self-care practices.

Mavacamten shows promise in treating adolescent obstructive hypertrophic cardiomyopathy, significantly improving heart function and safety in a pivotal trial.

Pharmacists play a crucial role in preventing OTC drug abuse through proactive interventions and effective warning measures, according to recent research findings.
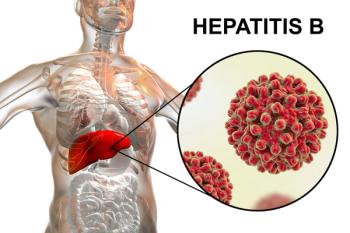
GSK announces promising phase 3 trial results for bepirovirsen, a potential treatment for chronic hepatitis B, showing significant functional cure rates.

Coping strategies significantly influence psychological health and functional capacity in COPD patients, highlighting the need for effective management.
COVID-19 vaccination significantly reduces long-term mortality risk in chronic obstructive pulmonary disease patients, highlighting its importance for respiratory health and safety.

FDA approves denosumab biosimilars Boncresa and Oziltus, enhancing access to affordable treatments for osteoporosis and oncology-related conditions.

Pharmacists are transforming reproductive health care by expanding their roles in contraception and patient support, despite facing significant barriers.

Orforglipron shows promise in maintaining weight loss for those transitioning from injectable GLP-1 therapies, offering a convenient oral alternative.

FDA approves tradipitant, a treatment for motion sickness, offering effective prevention after 40 years without new options.

Explore the latest insights on team-based care, AI in pharmacy, and innovative PBM models shaping the future of health care in 2025.

Pharmacy benefit manager reforms and new pain management options reshape the pharmaceutical landscape, enhancing patient access and care in 2025.

Pharmacists are expanding their roles in reproductive health, overcoming barriers to provide essential services and improve community care.
Published: September 2nd 2025 | Updated: September 5th 2025

Published: November 6th 2025 | Updated:

Published: August 25th 2025 | Updated:

Published: October 5th 2025 | Updated:

Published: August 1st 2025 | Updated:

Published: October 4th 2025 | Updated: