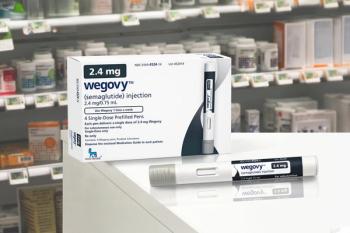
Semaglutide resulted in a significant reduction in the risk of MACE within the first 3 months of treatment compared to placebo.

Semaglutide resulted in a significant reduction in the risk of MACE within the first 3 months of treatment compared to placebo.

Vin Gupta, MD, discusses trends and perspectives in patient data privacy as well as what a “smart pharmacy” may look like in the near future.
Patients with psychosis, depression, and anxiety exhibit higher rates of vaccine hesitancy.

Greg Miller, RPh, discussed the past, present, and future of the Inflation Reduction Act and key insights pharmacists should understand in order to keep up with the legislation.

Cami Agena, Esq, and Candida Ruesga, Esq, discuss pharmacy benefit manager reform by providing insights from their experience as legal partners in complex PBM matters.
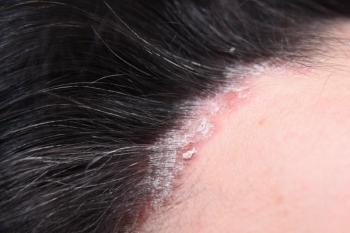
In a phase 3 study, 57% of patients treated with icotrokinra achieved an Investigator's Global Assessment score of 1 or 0 and a grade 2 or more improvement at week 16.
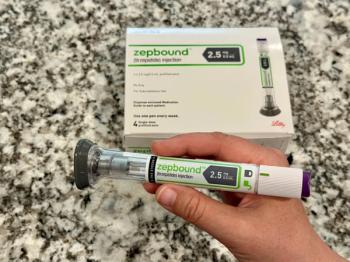
Tirzepatide (Zepbound) demonstrated superiority compared to semaglutide (Wegovy) across the primary end point and 5 key secondary end points.

Larry King, PharmD, discusses how pharmacy interoperability is working to create collaborative care and get pharmacists “in the game” as he says.

The order is set to make patients’ prescription costs similar to other nations with the lowest-priced drugs in the world.
Investigators find that unhealthy eating and inadequate or excessive sleeping duration could contribute to developing diabetic nephropathy.

Douglas Hoey, CEO of NCPA, discusses what independent pharmacies can do to handle an influx of patients and what the chain’s situation means for the pharmacy landscape.

The Summit provides a rare opportunity for professionals to dive deep into emerging healthcare technologies and treatment models.

Vin Gupta, MD, former Chief Medical Officer at Amazon Pharmacy and renowned US medical analyst, discusses pharmacists’ ability to reach communities and address social determinants of health.

Stanley V. Campbell, Jr., CEO of EagleForce Associates, Inc., and Yi Deng, PhD, Senior Vice President of Engineering at EagleForce, discuss the basics of AI and how it’s assisting with compliance.

The therapy achieved positive results during a phase 2b trial on the primary endpoint of percent change in Eczema Area and Severity Index (EASI) score.

Miranda Rochol, Senior Vice President of Provider Solutions at Prescryptive Health, discusses digital prescription solutions and how they are turning patients into consumers.
Brigid Groves, PharmD, MS, Vice President of Professional Affairs at APhA, discusses how the pharmacist’s role in immunization has adapted in recent history.
The therapy is currently under FDA review and has a PDUFA action date of May 22, 2025.

Angiotensin-converting enzyme inhibitors have been known to be more effective in decreasing the incidence of major adverse cardiac events for patients with diabetes.

President of 3 Axis Advisors and CEO of 46brooklyn Research joins Drug Topics to discuss nuances surrounding pharmacy benefit managers and the way they are perceived in the public eye.

A conversation with Douglas Hoey, RPh, MBA, CEO of NCPA, on the chain pharmacy’s announcement of a second Chapter 11 bankruptcy.
Guselkumab is the first and only IL-23 inhibitor to demonstrate robust data as a fully subcutaneous regimen.

Stanley V. Campbell, Jr., CEO of EagleForce Associates, Inc., and Yi Deng, PhD, Senior Vice President of Engineering at EagleForce, explore real-world examples of AI giving time back to pharmacists.

Patients often have anxiety about potential adverse effects, and experiencing adverse effects can affect patient adherence.
A phase 2 study found 95% of patients achieved a sustained virologic response at 12 weeks regardless of treatment adherence.

In this episode of the podcast, Natalie DiPietro Mager, PharmD, PhD, sits down with Jasmine Cutler, PharmD, CPh.

Selarsdi is interchangeable for all indications of ustekinumab, including treatment of psoriatic arthritis, plaque psoriasis, Crohn disease, and ulcerative colitis.

Stanley V. Campbell, Jr., CEO of EagleForce Associates, Inc., and Yi Deng, PhD, Senior Vice President of Engineering at EagleForce, discuss how AI is being used to benefit patients.

The use of electronic prescribing can also bridge the gap in clinical history, which can help pharmacists be aware of adverse drug reactions and medication interactions.

Among patients treated with buprenorphine, 25.4% experienced adverse pregnancy outcomes, compared to 30.8% in the untreated group.