Johnson & Johnson released a statement providing more information on the risks and benefits of its single-shot COVID-19 vaccine.

Johnson & Johnson released a statement providing more information on the risks and benefits of its single-shot COVID-19 vaccine.
Pharmacists reflect on their takeaways from the experience and how to move forward.

Administering pediatric immunizations can be a window of opportunity for pharmacists to demonstrate their value.

This article is sponsored by STChealth.

In part 2 of our interview, Jason Ausili, PharmD, speaks to the challenges of COVID-19 vaccination processes and dives into technology solutions for these workflow barriers.

There was a marked decline in child and adolescent routine vaccine administration during the COVID-19 pandemic between March and September 2020; these vaccination rates have continued to lag behind.
Novavax’s recombinant nanoparticle protein-based COVID-19 vaccine showed an overall efficacy of 90.4% and 100% efficacy against moderate and severe disease.

Here’s a preview of the featured content to watch for this week on Drug Topics®.

Moderna has filed for FDA emergency use authorization for use of its COVID-19 vaccine in adolescents aged 12 through 17.

The vaccine is indicated to prevent invasive disease and pneumonia caused by 20 Streptococcus pneumoniae serotypes.
Jason Ausili, PharmD, highlights President Biden's expectations for community pharmacies amid the COVID-19 vaccination effort.
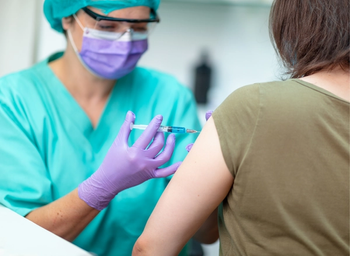
A study highlighted at the 2021 ASCO Annual Meeting explored whether patients who received an influenza vaccine were at increased risk for immune-related adverse events from single-agent immune checkpoint inhibitors.

Most pharmacists who participated in the survey also said they were comfortable addressing vaccine hesitancy with their patients.

Moderna has initiated the rolling submission process for the biologics license application for its COVID-19 vaccine mRNA-1273.
Vaccination activities build on collective success of COVID-19 response over the past year
The company has publicized data from the TeenCOVE study of the vaccine along with plans to seek authorization in the coming weeks.

The Practice Advisory, developed by the American College of Obstetricians and Gynecologists (ACOG), offers an overview of the latest guidance for COVID-19 vaccination in pregnant and lactating patients.
Here’s a roundup of the latest coronavirus-related news.

A research letter reported that robust secretion of SARS-CoV-2 specific IgA and IgG antibodies were found in breast milk of breastfeeding women for 6 weeks after the first of 2 vaccinations against COVID-19.
There is currently limited data on COVID-19 vaccine safety and efficacy in pregnant and lactating women.
The CDC today announced an updated guidance on mask wearing and social distancing for individuals fully vaccinated against COVID-19.

The FDA has expanded the emergency use authorization for the COVID-19 vaccine to include individuals 12 to 15 years of age.

Good Neighbor Pharmacy, AmerisourceBergen’s network of independent community pharmacies, launched a COVID-19 Vaccines Pharmacy Locator Tool on MyGNP.com.
Ease patients’ concerns by being well versed in the safety profiles of each vaccine.
Pfizer and BioNTech have initiated rolling submission of their Biologics License Application for full approval of BNT162b2.