
The federal agencies said that they will continue to work closely with manufacturers to maintain the availability of the vaccines through the end of 2023 and early 2024 to meet the demand during the RSV season.

The federal agencies said that they will continue to work closely with manufacturers to maintain the availability of the vaccines through the end of 2023 and early 2024 to meet the demand during the RSV season.

Unlike traditional THC, which is federally banned, D8 has managed to find a legal loophole, making it widely accessible.

Implementing a standardized oxytocin infusion protocol may increase patient safety while reducing maternal adverse effects that are associated with rapid, high rates of infusion of oxytocin.
The REFLECT Scoliosis Correction System from Globus Medical is intended to correct progressive scoliosis in pediatric patients while simultaneously maintaining stability and motion as well as allowing for future modulated growth.

The FDA has finally decided to withdraw approval of Makena and its generics after Covis Pharma volunteered to remove the drug from market in March.

Results from a phase 3 study show that the drug is effective in treating VMS symptoms due to menopause.
The drug, manufactured by Sandoz, will treat conditions including osteoporosis in postmenopausal women

A single oral dose of azithromycin during labor in women planning vaginal delivery can significantly reduce the risk of maternal sepsis or death
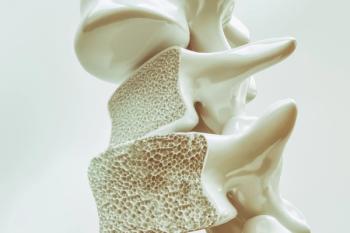
The Biologics License Application of a proposed biosimilar of denosumab, a drug that could treat several bone diseases and issues, has been accepted by the FDA.
Several factors predict itch-specific quality of life in patients with chronic itch.

This is the first FDA-approved ADC for platinum-resistant disease, according to ImmunoGen.

History of migraine may be associated with poor sleep in premenopausal and perimenopausal women, according to research presented at the North American Menopause Society Annual Meeting.
The FDA has granted Emergency Use Authorization EUA of the Jynneos vaccine for pediatric patients <18 years who are at high risk of monkeypox infection.

Myovant Sciences and Pfizer announces the FDA has approved the therapy for the once-daily treatment of moderate-to-severe endometriosis-associated pain.

Perrigo’s HRA Pharma has submitted an application to the FDA for possible future approval of the first-ever OTC birth control pill.
Oteseconazole (Vivjoa) reduces the incidence of recurrent vulvovaginal candidiasis.
"The spread of misinformation and mistrust in doctors and science is contributing to staggeringly low vaccination rates among pregnant people," said the American Colleges of Obstetricians and Gynecologists.

A recent test found various brands and batches of sunscreen and after-sun care products contained benzene, a potential carcinogen.
Increasing reports have surfaced of vaccine recipients experiencing delayed cutaneous side effects after receiving the first dose.

Published: May 27th 2021 | Updated:

Published: March 7th 2023 | Updated:

Published: July 11th 2022 | Updated:
Published: March 23rd 2021 | Updated:

Published: March 15th 2023 | Updated:

Published: March 8th 2023 | Updated: