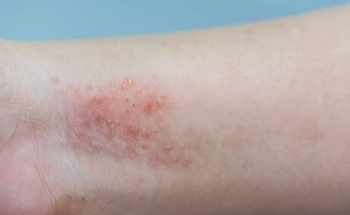
The FDA approved the topical JAK1/JAK2 inhibitor ruxolitinib for the treatment of mild to moderate atopic dermatitis in September 2021.

The FDA approved the topical JAK1/JAK2 inhibitor ruxolitinib for the treatment of mild to moderate atopic dermatitis in September 2021.
One of the key differences between diagnosing AD in patients with skin of color compared to White skin is erythema.

Targeting skin care regimens to the individual patient is most likely to yield compliance to a recommended routine.
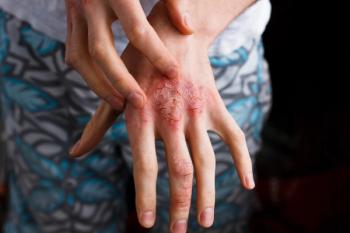
Studies offer positive outcomes for patients with conditions like hidradenitis suppurativa or psoriasis.
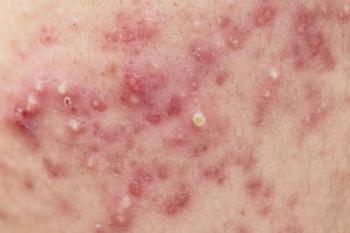
As skin manifestations are commonly the first thing spotted with infectious disease, dermatologists are in a great position to counsel patients about prevention.
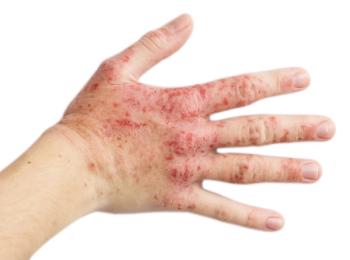
AbbVie announced the approval after 3 studies show the efficacy and safety of upadacitinib using mono- and combination therapy.

A national survey found that patients are willing to try medical cannabis products as potential treatments of their condition. Additionally, some already use over-the-counter cannabis products.

The FDA approved Cosentyx (secukinumab) to treat moderate-to-severe plaque psoriasis in children aged 6 years and older.

A recent test found various brands and batches of sunscreen and after-sun care products contained benzene, a potential carcinogen.

Tremfya phase 3 data shows skin clearance rates were maintained at 5 years with 55.5% of patients achieving an IGA score of 0% and 53% achieving PASI 100 response in VOYAGE 2 trial.

Tirbanibulin (Klisyri; Almirall) is indicated for the topical treatment of actinic keratosis on the face or scalp.
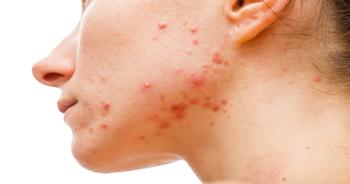
Clascoterone cream 1% is a first-in-class topical acne treatment that targets the androgen receptors in the skin.

Study suggests higher hospitalization rate for some skin diseases.

Dupilumab (Dupixent, Sanofi and Regeneron) is the first biologic medicine approved for treatment of moderate-to-severe atopic dermatitis.
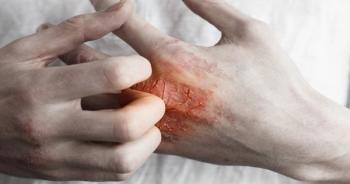
Apremilast (Otezla, Amgen) significantly improved psoriasis symptoms compared with a placebo in adults with mild-to-moderate disease.

Crisaborole ointment, 2% (Eucrisa, Pfizer) has been approved for use in pediatric patients with atopic dermatitis as young as 3 months of age.
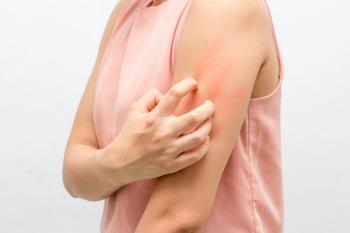
Phase 3 clinical data demonstrate abrocitinib’s efficacy and safety in treating moderate-to-severe atopic dermatitis in adults.

Baricitinib (Olumiant, Eli Lilly and Incyte) demonstrated positive results in the BREEZE-AD5 study of patients with atopic dermatitis.

If approved, dupilumab (Dupixent, Regeneron and Sanofi) would be indicated for use as an add-on maintenance treatment for children ages 6 to 11 years with moderate-to-severe atopic dermatitis.

Pharmacists can play an integral role in managing patients with psoriasis through medication therapies and lifestyle modifications.
Available for prescribing starting January 2020
Originally approved in 1995 for use in adults only.

First new treatment of its kind in over 20 years.

Psoriasis, MS, Lupus, and RA.

TNF blocker biosimilar to Humira (adalimumab, Abbvie).