
Sanofi and Regeneron Pharmaceuticals, Inc announced the FDA approval of dupilumab. It is now the first biologic medicine for children from 6 months to 5 years old with moderate to severe atopic dermatitis.

Sanofi and Regeneron Pharmaceuticals, Inc announced the FDA approval of dupilumab. It is now the first biologic medicine for children from 6 months to 5 years old with moderate to severe atopic dermatitis.

Regeneron Pharmaceuticals Inc came to an agreement to buy the drug from Sanofi for up to $1.1 billion.

UCB announced the FDA issued a CRL that stated that the FDA cannot approve the BLA in its current form.

Ruxolitinib cream 1.5% may soon be the first FDA-approved treatment for repigmentation of vitiligo. Experts share what dermatologists need to know regarding efficacy, safety, and cost of this topical drug.

An American Academy of Dermatology survey found that more Americans say sun protection is more important than 5 years ago, but don’t understand how to protect themselves.
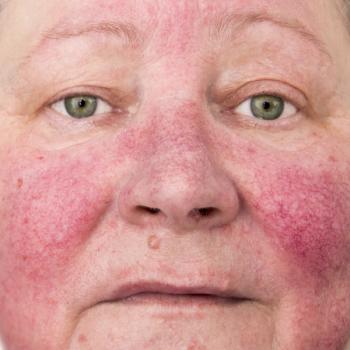
Sol-Gel Technologies and Galderma announced the FDA approval of the 5% benzoyl peroxide, cream for the treatment of inflammatory lesions of rosacea.
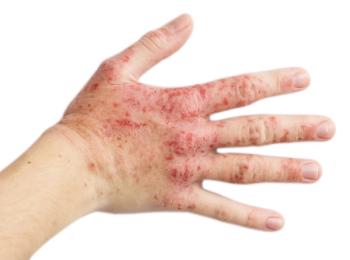
A supplemental biologics license application was recently submitted.
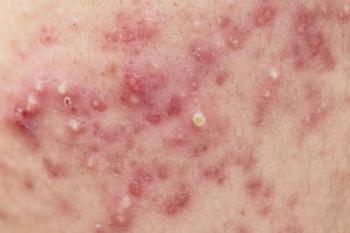
A recent study investigated the potential adverse events around using 5-aminolaevulinic acid photodynamic therapy (ALA-PDT) for acne treatment.
A recent study investigated how the limited access to specialty care in these populations may affect the detection and diagnosis of skin cancer.

The American Academy of Dermatology’s newest guideline focuses on awareness of other health conditions associated with atopic dermatitis in adult patients.
A retrospective cohort study in a research letter published in the Journal of the American Academy of Dermatology examined the risk between atopic dermatitis and COVID-19 risk.
A study published in the Journal of the American Academy of Dermatology did a prospective analysis on psoriasis biologic treatment response related to comorbid obesity and a history of diabetes.

A national survey found that patients are willing to try medical cannabis products as potential treatments of their condition. Additionally, some already use over-the-counter cannabis products.
IDP-126, an acne vulgaris treatment for patients ages 9 and older, met all of its endpoints in a 12-week phase 3 study.
Published: September 23rd 2021 | Updated: