
Test your pharmacy news knowledge with our weekly quiz.

Test your pharmacy news knowledge with our weekly quiz.

Spring and summer not only bring sunshine, but also stronger UV rays. Whether you are barbecuing, swimming, or taking a hike, skin protection is paramount while soaking up the sunshine.

Recent research exploring the gut-skin connection shows that dietary modifications and probiotic supplements may help skin conditions like acne, atopic dermatitis, and rosacea.

The ADORING 3 phase 3 development program is currently studying the novel, aryl hydrocarbon receptor agonist as a once-daily, cosmetically elegant, and steroid-free topical cream for adults and children with atopic dermatitis.

Abeona Therapeutics’ pz-cel is an investigational autologous, COL7A1 gene-corrected epidermal sheet therapy for the treatment of recessive dystrophic epidermolysis bullosa.

Christopher Bunick, MD, PhD, FAAD, an associate professor of dermatology and a physician-scientist at the Yale School of Medicine in New Haven, Connecticut, presented updates on benzene in BPO at the 2024 American Academy of Dermatology Annual Meeting.

The average US household spent $645 on OTC products in 2022.
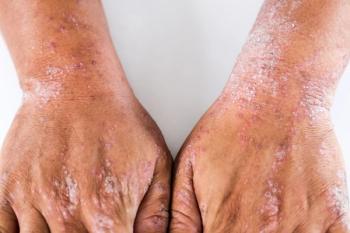
A lack of diversity in clinical trials has hampered health care providers from understanding the nuances of diseases across specific patient populations.

Following investigation by the independent testing laboratory, Valisure found acne products that contain up to 12 times the allowed of benzene.

In 2017, dupilumab became the first treatment approved in Canada for moderate to severe AD, as multiple clinical trials demonstrated its effectiveness and safety.

The FDA's decision represents a landmark approval of a tumor-derived cellular therapy in a solid tumor cancer.

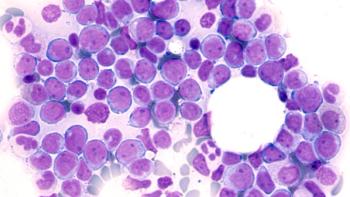
Biologic approvals ranged from gene therapies for hemophilia A to a cellular therapy to treat type 1 diabetes.

In addition to the numerous adalimumab (Humira) biosimilars that launched this year, multiple new biosimilar products were approved by the FDA in 2023.

Investigators recommended that isotetinoin be used with extreme caution among patients with a history of mental illness in light of study findings.

Tralokinumab is the first form of atopic dermatitis treatment that targets the interleukin-13 cytokine, the main source of atopic dermatitis symptoms.

The foam formulation and once-daily application of roflumilast foam 0.3%, the first FDA-approved drug for seborrheic dermatitis with a new mechanism of action in over 2 decades, makes it a favorable alternative to existing treatments.

The treatment from Arcutis Biotherapeutics has a Prescription Drug User Fee Act (PDUFA) target action date of July 7, 2024.
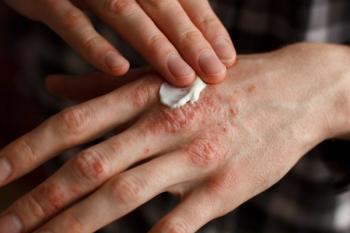
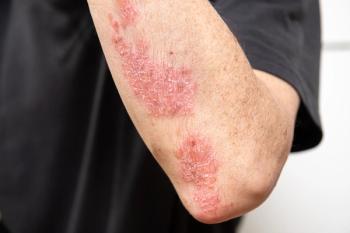
Findings from the study highlight potential unmet needs had by patients with mild psoriasis seeking treatment.
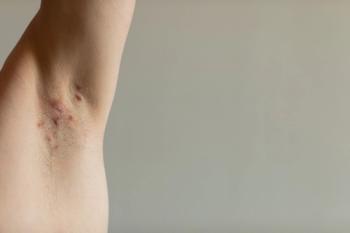
Secukinumab is the only anti IL-17A inhibitor approved for hidradenitis suppurativa.
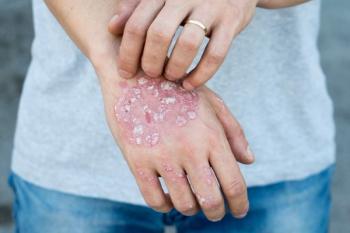
Ustekinumab-auub is approved for use in both adult and pediatric populations.

