
Researchers conduct a review to understand the efficacy and safety of topical minocycline for the treatment of moderate to severe papulopustular rosacea.

Researchers conduct a review to understand the efficacy and safety of topical minocycline for the treatment of moderate to severe papulopustular rosacea.

The subgroup analysis shows adolescents treated with the drug once daily had higher rates of clear or almost clear skin compared to patients receiving the placebo.

Over 20 years since skin of color dermatology was introduced, researchers explored advancements and what is yet to come in the future.

The FDA approved adalimumab-aaty in May 2023 for 8 indications, including rheumatoid arthritis, psoriatic arthritis, Crohn disease, ulcerative colitis, and plaque psoriasis.

Researchers explored TikTok’s role in the dissemination of information regarding isotretinoin and its users.

In a comprehensive review, researchers aimed to explore seasonality, environmental factors, and the genetic and epigenetic mechanisms of patients living with psoriasis vulgaris.

In a review of skin inflammation’s link to food allergen sensitivity, researchers explored how patients with atopic dermatitis may be more susceptible to the development of food allergies.

Patients treated with the therapy showed significantly less progression of structural damage compared to patients who received placebo at week 24.
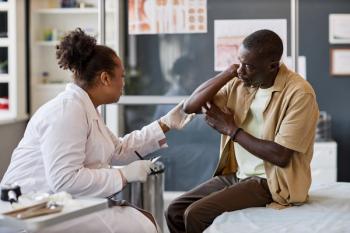
Researchers explored how entrustable professional activities can revolutionize workplace-based training and evolve the role of pharmacists within dermatology clinics.
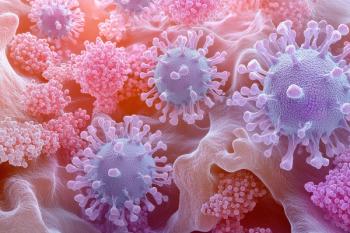
Researchers conducted a comprehensive review of allergic contact dermatitis, detailing the condition’s epidemiology, pathophysiology, clinical manifestations, diagnostic approaches, and therapeutic options.

Xyngari resulted in significant improvements in IGA treatment success, inflammatory lesion count, and non-inflammatory lesion count compared to placebo.

Deucravacitinib (Sotyktu) also demonstrates improvements in signs and symptoms, extra-articular manifestations, and patient-reported outcomes.
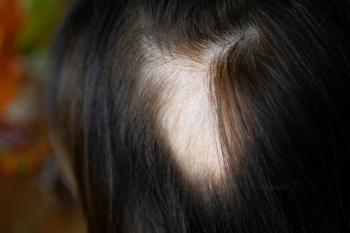
Patients aged 12 to under 18 saw clinically meaningful regrowth on the scalp, eyebrow, and eyelashes with both high-dose and low-dose treatment.
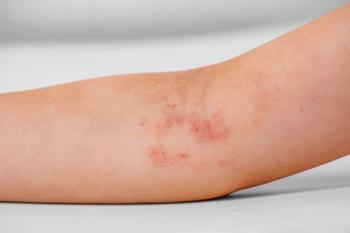
Investigators note that low itch and mild disease severity are maintained following a treatment-free interval of 80 days.
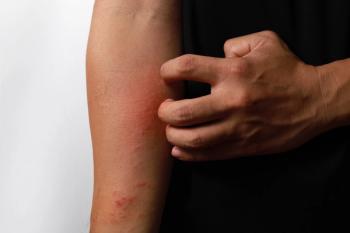
The ADjoin long-term extensive study is designed to assess the long-term safety and efficacy of lebrikizumab for moderate-to-severe atopic dermatitis.
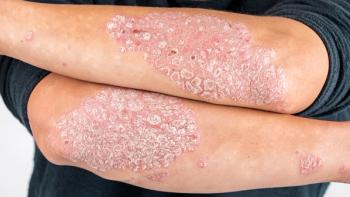
Investigators report that the safety profile is also similar between both drugs.
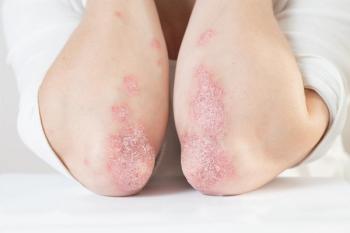
The FDA initially approved deucravacitinib (Sotyktu) in September 2022 to treat moderate-to-severe plaque psoriasis.
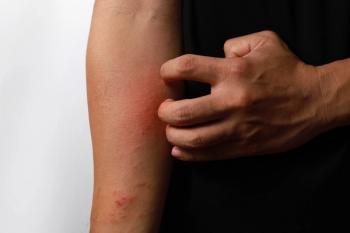
For patients with atopic dermatitis reporting moderate-to-severe scratching, researchers explored the efficacy of an AI-enabled wearable sensor with closed-loop haptic feedback.

Data from a previous phase 1b trial show improvements in the Eczema Area and Severity Index (EASI)-75 compared with the placebo at 12 weeks.

Researchers conducted a literature review of patients’ clinical experiences with using secukinumab to treat psoriatic arthritis.

Vtama from Organon was previously approved in May 2022 to treat plaque psoriasis in adults.
Ethan Melillo, PharmD, CDOE, senior manager of Integrated Health at L’Oréal, provides some of his favorite recommendations to help with irritated skin.
FDA approval was granted on September 13, 2024.

The agency assigned prademagene zamikeracel a PDUFA target action date of April 29, 2025.
“Emrosi has potential to become the best-in-class oral medication to treat the condition,” said the CEO of Journey Medical.