Articles by Kaitlyn Bader

Christopher Bunick, MD, PhD, FAAD, an associate professor of dermatology and a physician-scientist at the Yale School of Medicine in New Haven, Connecticut, presented updates on benzene in BPO at the 2024 American Academy of Dermatology Annual Meeting.

Following investigation by the independent testing laboratory, Valisure found acne products that contain up to 12 times the allowed of benzene.

The foam formulation and once-daily application of roflumilast foam 0.3%, the first FDA-approved drug for seborrheic dermatitis with a new mechanism of action in over 2 decades, makes it a favorable alternative to existing treatments.

The news comes almost 2 weeks after Cyltezo’s entrance into the US biosimilars market as the first commercially available and FDA-approved interchangeable adalimumab biosimilar.

Boehringer Ingelheim’s Cyltezo is the first and only FDA-approved interchangeable biosimilar to adalimumab.

Brett King, MD, PhD, gave an in-depth overview of how JAK inhibitors earned a boxed warning and what it means for treating patients with atopic dermatitis at RAD 2023.

The expected PDUFA date is December 16, 2023.
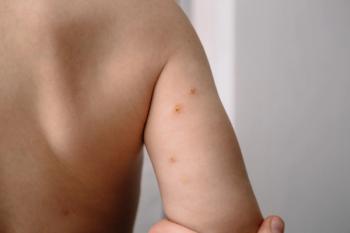
VP-102 hopes to treat molluscum contagiosum, pending approval from FDA
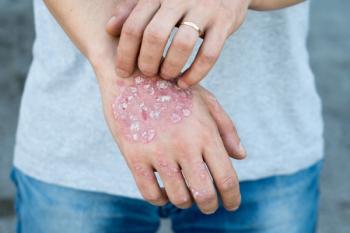
Linda Stein Gold, MD explains in nonsteroidal treatment options for plaque psoriasis.
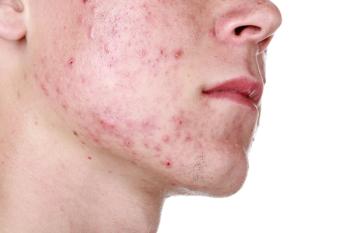
The relationship between dermatology and social media was analyzed in a poster presentation during SBS 2023 in Miami, Florida.

Natasha A. Mesinkovska, MD, PhD, shares what’s new in alopecia areata management.

Understanding the relationship between psoriasis and psoriatic arthritis can help.

Immune checkpoint inhibitor therapy does not appear to increase complication
rates in patients undergoing dermatologic procedures.
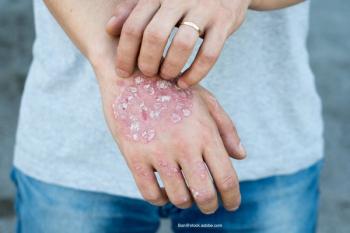
BLA submission is based on phase 3 trial results evaluating the safety and efficacy of lebrikizumab.
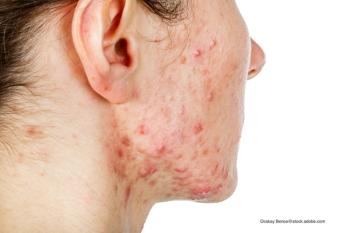
Numerous adverse effects are associated with standard-dose isotretinoin.
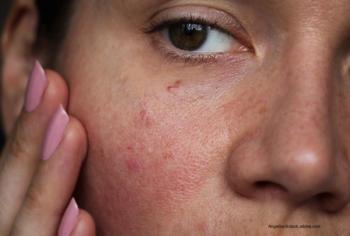
State of Skin Sensitivity Report raises awareness of the causes of skin sensitivity and its effect on the mind.
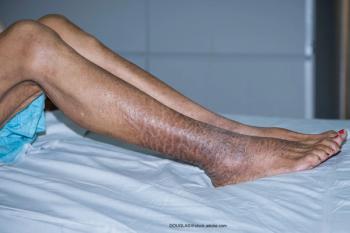
Timber Pharmaceuticals’ CONTROL study evaluated the safety and efficacy of the topical isotretinoin.

Janssen Pharmaceutical Companies of Johnson & Johnson announced the approval of ustekinumab as a new treatment option for patients aged 6 years or older with active psoriatic arthritis.

Arcutis Biotherapeutics’ roflumilast cream will provide relief to patients aged 12 years or older diagnosed with plaque psoriasis.

Ruxolitinib (Opzelura) cream is the first and only FDA-approved treatment for patients with vitiligo.

Perimenopausal, menopausal, and postmenopausal women experienced a progressive improvement in hair growth, shedding, quality of life, and menopausal symptoms after 12 months with an active nutraceutical supplement.

























