
Researchers were unable to find a significant benefit for patients for the majority of cancer medications given accelerated FDA approval.

Researchers were unable to find a significant benefit for patients for the majority of cancer medications given accelerated FDA approval.

Overall, older adults with diabetes faced a 53% higher risk of developing at least 1 functional limitation during the pandemic compared to older adults without diabetes.

A Q&A with Douglas Hoey, CEO of NCPA, on how the new provision from CMS requiring DIR fees at the point of sale is impacting independent pharmacies in 2024.

The ongoing opioid crisis in the United States shows no signs of slowing down. As trusted community health care professionals, pharmacists must play a key role in fighting it.

With an unprecedented number of Americans in need of mental health care, what role should pharmacists play when it comes to screenings?

With the judge’s failure to approve a settlement class, a $13.5 million settlement against Eli Lilly was called off.
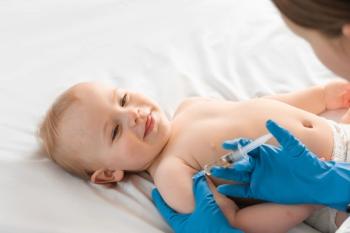
Findings indicating that socioeconomic disparities widened gaps in vaccination timeliness signal the need for increased efforts to promote timely vaccination among children from families with lower income and those without private insurance.
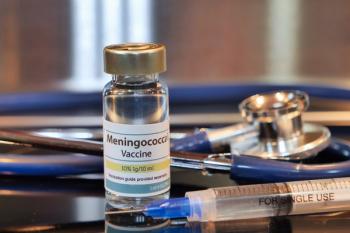
The company’s investigational ABCWY vaccine candidate will be reviewed by the FDA by February 14, 2025.

April showers might bring May flowers, but for some, the month also brings the return of allergy woes. This spring, breathe easy with these OTC allergy product recommendations.

The DEA informed community pharmacists on e-prescribing fraud and measures necessary to detect it.
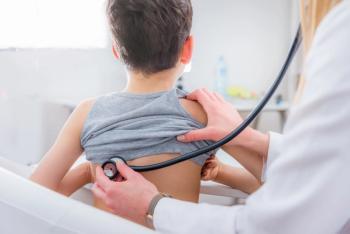
A study from the Children’s Hospital of Philadelphia found that a positive PCR test was not associated with an increased risk of a new asthma diagnosis in patients ages 1 to 16.

A string of thefts at local pharmacies has spurred the DEA to issue warnings and share tips for independent pharmacists to keep their stores secure.

See what's trending in pharmacy with a preview of the Total Pharmacy April issue.
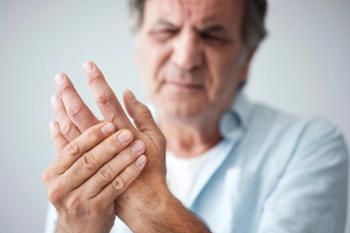
In the United States, 12 million adults suffer from co-occurring chronic pain and anxiety or depression. Erasing stigmas, educating patients, and tailoring interventions can help to improve outcomes.
Drug Topics sat down with Sally Rafie, founder of Birth Control Pharmacist, to discuss how pharmacists are well positioned to expand access to reproductive care.

Check out a recap of important pharmacy news you might’ve missed this week, dispensed in small doses.

After a long hiatus, research in psychedelic medicine is progressing rapidly. With the potential approval of MDMA in the near future, what role will pharmacists play in integrating these new therapies into treatment plans?
Since a 2019 measles outbreak, the CDC has seen an increasing number of cases in the US.
Although over 38 million people in the US have diabetes, continuous glucose monitor (CGM) uptake is low. Community pharmacists are uniquely positioned to promote uptake due to their frequent interactions with patients and expertise in chronic condition counseling.

The CDC addressed an HPAI A(H5N1) virus infection reported by a commercial dairy farmer in Texas.

Governor Glenn Youngkin vetoed bills HB570 and SB274 that would have allowed the state to establish its first Prescription Drug Affordability Board.

“Every pharmacist raises their hand and takes an oath before they graduate to put patients first,” said Bruce Berger, PhD, FAPhA. “But what happens when you exist in an environment every single day where that’s impossible?”

Japan’s health ministry has issued a warning to consumers regarding red yeast rice dietary supplements containing a red species of mold after 5 people died and more were hospitalized after consumption.

See what's trending in pharmacy with a preview the Drug Topics April issue.

Researchers addressed pharmacists’ role in mitigating drug shortages.

Due to the increased possibility of adverse pregnancy outcomes associated with methotrexate and leflunomid, it is crucial that patients with rheumatoid arthritis (RA) engage in thorough consultations with their physicians regarding pregnancy planning.

Check out a recap of important pharmacy news you might’ve missed this week, dispensed in small doses.

With drug price negotiations for 10 drugs through the Inflation Reduction Act (IRA) set to take effect in 2026, researchers simulated the extent of Medicare savings.
In 4 posters presented at the Hematology/Oncology Pharmacy Association Annual Conference 2024, researchers addressed pharmacists’ role in managing patients’ anticancer medications.

Vistagen has enrolled the first participant in the PALISADE-3 phase 3 trial of fasedienol for the treatment of social anxiety disorder.