The study found that genetic variability across HLA A, B, and C could affect susceptibility to and severity of COVID-19.

The study found that genetic variability across HLA A, B, and C could affect susceptibility to and severity of COVID-19.

If health care were a country, it would be the fifth largest emitter of greenhouse gases worldwide.

This is the first diagnostic test authorized by the FDA with a home collection option for COVID-19.

The guidelines provide clinical recommendations to providers treating patients with coronavirus disease 2019 (COVID-19).

"With the introduction of this ibrutinib-rituximab combination, patients now have a more effective, non-chemoimmunotherapy option."

“The reality is that pharmacy workers are smack dab in the middle of trauma.”
Early treatment of shingles reduces symptoms and risk of serious complications.


Eighty-eight percent of community pharmacy owners reported intentions to apply for COVID-19 federal aid, such as provisions from the CARES Act, according to the survey

The study examined the effects of unprotected, prolonged patient contact, along with certain exposures, on the risk of health care providers becoming infected with COVID-19.

Genentech is seeking approval for a 2-hour ocrelizumab (Ocrevus) infusion time in patients with relapsing or primary progressive multiple sclerosis.

Officials with the FDA today approved tucatinib (Tukysa, Seattle Genetics) in combination with chemotherapy trastuzumab and capecitabine for the treatment of adults with advanced forms of HER2-positive breast cancer.

Here's a roundup of the latest coronavirus-related news.
Investigators used simulation models to project the transmission dynamics of COVID-19.

President Donald J. Trump announced his proposed plan for reopening US state economies amidst the coronavirus disease 2019 (COVID-19) pandemic, with steps outlined in a 3-phase approach.

“Pharmacists are the safety net for their communities…we must ensure that these pharmacies have access to the tools they need to fight this pandemic and continue being there for patients in the future.”
Insulin manufacturers are expanding insulin affordability options for patients with diabetes during the coronavirus pandemic.
In 2 prior clinical studies, gimsilumab demonstrated favorable results in treating the novel coronavirus.

A recent meta-analysis showed an association between regular aspirin use and reduced risk of developing several cancers of the digestive tract.

The American Heart Association’s scientific statement outlines medications, procedures, and lifestyle change recommendations for individuals with both type2 diabetes and coronary artery disease.

Merck has announced the US launch of trastuzumab-dttb (Ontruzant), a biosimilar to trastuzumab (Herceptin), in both 150-mg single-dose vials and 420-mg multiple-dose vials.

As the global COVID-19 pandemic and consequent public health crisis continues on, health care providers on the frontlines of this crisis deserve all of the support needed to continue serving their patients.
GlaxoSmithKline and Sanofi have prioritized public health over competition in their agreement, announced today.
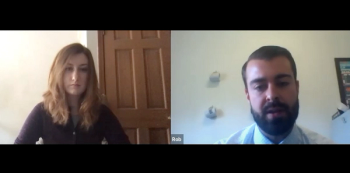
In part 2 of our video interview, Robert Brunault, PharmD, a clinical pharmacy specialist in hematology and oncology at Rhode Island Hospital and the Lifespan Cancer Institute, discussed drug shortages, FDA emergency use authorizations, and the biggest concerns for pharmacists.

Gilead shares compassionate use data for remdesivir in patients with COVID-19 and a new research paper suggests remdesivir's mechanism of action could make it effective in combatting the virus.

In an opinion piece published in the Annals of Internal Medicine, community pharmacists were credited with helping to mitigate the spread of coronavirus throughout Taiwan.

“You’re basically on call 24/7, 365 days a year. That’s the biggest word of caution I would offer to someone considering this. But at the same time, the biggest reward I get is seeing sick people get better."

In collaboration with Spectrum Solutions and Accurate Diagnostic Labs, RUCDR Infinite Biologics developed the new collection approach, which utilizes saliva as the primary test biomaterial for COVID-19.

This year may mark a dramatic difference in the development and approval of complex generic drug products.