
A survey from APhA reported that the majority of pharmacists plan to or have received a COVID-19 vaccine.

A survey from APhA reported that the majority of pharmacists plan to or have received a COVID-19 vaccine.

In its next live webinar event, MJH Life Sciences™ COVID-19 Coalition will explore emerging mutations of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).

Drug Topics interviews Naomi Lopez, director of healthcare policy at the Goldwater Institute, focusing on expanding scope of practice and COVID-19 vaccine candidates already being used in other countries.

In a letter to President Joe Biden, ASHP and more than 50 signers called for use of federal resources and expansion of the vaccinator workforce to confront the slow ramping up of COVID-19 vaccinations.
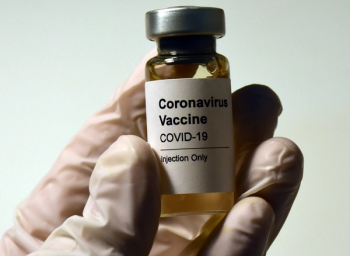
ISMP identified the most commonly reported errors and hazards associated with the new COVID-19 vaccines.

President-elect Joe Biden revealed his COVID-19 plan, which includes a disciplined focus on vaccination efforts.
Here's the latest coronavirus-related news.
On October 22, 2020, the FDA approved remdesivir (Veklury; Gilead) as the first treatment for coronavirus disease 2019 (COVID-19).

Amid the pandemic, how effectively pharmacists embraced their role as frontline care providers could change the profession forever.

In a final briefing, OWS recommended states expand vaccination to all individuals 65 and older or those with comorbidities.
The FDA has identified 3 molecular tests that may be impacted by an emerging SARS-CoV-2 variant.

Here’s a roundup of the latest coronavirus-related news.

In a briefing on Wednesday, Dr Hanson and Dr Caliendo of the Infectious Diseases Society of America relayed the updated recommendations for COVID-19 diagnostic testing.

How will a change in administration, a vaccine for COVID-19, and other factors change the industry in the year ahead?

Moderna is upping its COVID-19 vaccine production this year, with a baseline estimation of 600 million doses for 2021.

In a statement, FDA officials recommended that all health care professionals continue to adhere to the FDA-authorized dosing and schedules of the COVID-19 vaccines.

With support from Operation Warp Speed and the National Institutes of Health, the study will evaluate the safety and efficacy of NVX-CoV2373.

Eric Levin, CEO of Scripta Insights, discusses trends that are shifting pharmacy toward delivery services and the impacts on the prescription drug market.

Stephen Schondelmeyer, PharmD, Drug Topics editorial advisory board member, discusses pharmacy highlights from 2020, and where he sees pharmacy headed in 2021.

Here are some of the top-read FDA approvals covered by Drug Topics® from this year.

Ken Thai, PharmD, Drug Topics editorial advisory board member, lists the key trends from 2020 and where he envisions pharmacy going in 2021.

Lakesha Butler, PharmD, Drug Topics editorial advisory board member, examines 2020 trends in diversity, equity and inclusion within health care and explores the year ahead.

Cody Clifton, PharmD, CPESN USA, discusses the organization's pharmacy integration network and the pharmacists' role amid the coronavirus pandemic.
The Drug Topics® team rounded up some of our top-read articles from the last year.

Here’s the last roundup of the latest coronavirus-related news in 2020.