
Guest host Brian Nightengale, president of Good Neighbor Pharmacy, is joined by Rich Tremonte and Doug Hoey to discuss the biggest opportunities and headwinds facing independent pharmacies in 2021.

Guest host Brian Nightengale, president of Good Neighbor Pharmacy, is joined by Rich Tremonte and Doug Hoey to discuss the biggest opportunities and headwinds facing independent pharmacies in 2021.

MJH Life Sciences™ COVID-19 Coalition will be hosting its next live webinar event “Thinking Long-Term: COVID-19’s Clinical Consequences and Mental Health Effects."

Members of the MJH COVID-19 Coalition were surveyed about strategies for pandemic containment.

The UK study examined the cross-sectional association between vitamins A, E, C, and D and respiratory complaints.

Here’s a preview of the featured content to watch for this week on Drug Topics®.
The recent uptick in antibiotic use interrupted a steady 4-year downward trend, according to study authors.
Robert Hopkins, chair of the National Vaccine Advisory Committee, discusses the most pressing issues coming out of the recent NVAC meeting and pharmacy's role amid the COVID-19 pandemic.

Regeneron’s REGN-COV2 met primary and key secondary end points in its phase 2/3 trial evaluating the drug in the COVID-19 outpatient setting.

Robert Hopkins, chair of the National Vaccine Advisory Committee, discusses the most pressing issues coming out of the recent NVAC meeting, and pharmacy's role amid the COVID-19 pandemic.

CMS’ new plan will ensure coverage of COVID-19 vaccines with no out-of-pocket costs, while increasing the number of providers that will administer the vaccine.
Here's the coronavirus-related news from this week that you should know.

For our first episode in the ThoughtSpot 2020 series, experts from AmerisourceBergen and Healthcare Ready discuss how to support marginalized communities amid pandemics and natural disasters.

Although the company has ended the ACTIV-3 clinical trial, researchers are continuing to pursue all other studies of bamlanivimab in COVID-19.

Investigators stressed that governments, public health officials, and other non-governmental organizations should aim to build trust in a COVID-19 vaccine.

Here's the coronavirus-related news from this week that you should know.

The combined landscape of flu and COVID-19 presents challenges to vaccine uptake.

Remdesivir (Veklury; Gilead) is the first and only fully FDA-approved treatment for COVID-19.
The new guidance provides clarification on requirements for pharmacy interns and technicians to administer childhood and COVID-vaccines, as well as COVID-19 tests.
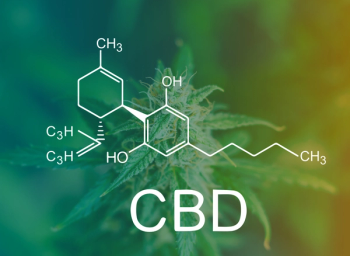
CBD and its impacts on a protective peptide show that the cannabinoid may reduce lung inflammation and damage.
The partnership aligns with Operation Warp Speed’s goal to protect the most vulnerable to COVID-19.

Here's the coronavirus-related news from this week that you should know.
In an open letter, Pfizer Chairman and CEO wrote that safety and efficacy data for its COVID-19 vaccine candidate may be ready by the third week of November for FDA submission.

The study will determine whether certain approved therapies or investigational drugs warrant advancement into larger-scale clinical trials for COVID-19.

AstraZeneca's investigational long-acting antibody combination will advance into phase 3 trials.

Here's the roundup for this week's coronavirus-related news.