
As a dozen Humira Biosimilars are set to enter the market this year, it seems to be unlikely that they will drive down drug costs in the future.

As a dozen Humira Biosimilars are set to enter the market this year, it seems to be unlikely that they will drive down drug costs in the future.

Pharmacists are well positioned to address the care issues faced by patients living with autoimmune diseases.
While the use of combined oral contraceptives in patients with rheumatoid and autoimmune disorders have focused on safety, a recent narrative review highlighted the efficacy and benefits of these contraceptives in women with RA.

Managing autoimmune conditions like type 1 diabetes, rheumatoid arthritis, and lupus during pregnancy can present challenges for patients and physicians.

Healthcare professionals need to make patients the primary decision-makers in their own care and actively support their efforts to achieve their care goals.
Emerging evidence is changing health care providers’ approach to treating pediatric rheumatic diseases.

Investigators supplemented a slim body of existing research on CBD for pain management.

A study presented at ACR Convergence 2021 examined the relationship between race/ethnicity, living in a disadvantaged neighborhood, and how often patients are prescribed disease-specific treatments.
Drug Topics® sat down with Dr. Daniel Streetman, PharmD, to discuss patients with compromised immune systems and reported responses to COVID-19 vaccines and others.
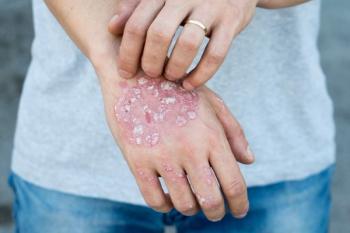
Pharmacists can educate patients on psoriasis treatments and lifestyle modifications, address expected drug-related adverse effects (AEs), and improve medication adherence.

Anifrolumab (Saphnelo; AstraZeneca) significantly improves disease activity and reduces corticosteroid use in patients with SLE.

Individuals with irritable bowel syndrome with constipation indicated that although their symptoms worsened during the pandemic, they did not seek medical care.
Pharmacists must keep up to date with the latest in irritable bowel syndrome management.

At the virtual Scientific Sessions for the American Diabetes Association, a study shows that metformin can improve adolescent type 1 diabetes.

A study presented during a poster session at the American Diabetes Association Virtual 81st Scientific Sessions evaluated performance, safety, and satisfaction of an extended-wear insulin infusion set in patients with type 1 diabetes.
Aducanumab (Aduhelm; Biogen) is the first approved novel treatment for Alzheimer disease since 2003.

Exposure to heat and sun can worsen symptoms for certain conditions.
Pharmacists can play an important role as part of an interdisciplinary team in treating patients with rheumatoid arthritis.

Tremfya phase 3 data shows skin clearance rates were maintained at 5 years with 55.5% of patients achieving an IGA score of 0% and 53% achieving PASI 100 response in VOYAGE 2 trial.

Research presented at Academy of Managed Care Pharmacy’s 2021 virtual conference evaluated tolerance and adherence to oral disease-modifying therapies for relapsing-remitting multiple sclerosis.


AbbVie submitted an application to the FDA for the use of risankizumab-rzaa, 150 mg (Skyrizi) in adults with active psoriatic arthritis.
Ponesimod was shown to be superior to teriflunomide in the rate of confirmed relapses and the number of new or enlarged T1 and T2 lesions.

Pharmacists can play an important role as part of the interdisciplinary care team.
Preliminary findings suggest patients with multiple sclerosis who are taking cladribine can mount and maintain effective vaccine responses.