
Discover critical insights on respiratory health, COVID-19 impacts, and vaccination strategies from recent studies and regional policy changes.

Laura Joszt, MA, is the vice president of content for the managed care and pharmacy brands at MJH Life Sciences®, which includes The American Journal of Managed Care®, Drug Topics®, Managed Healthcare Executive®, and Pharmacy Times®. She has been with MJH Life Sciences since 2011.
Laura has an MA in business and economic reporting from New York University. You can connect with Laura on LinkedIn or Twitter.

Discover critical insights on respiratory health, COVID-19 impacts, and vaccination strategies from recent studies and regional policy changes.

OTC access expands, enhancing reproductive health and migraine relief while raising safety concerns about online pharmacies and medications like Benadryl.
The top pneumococcal stories of 2025 explored the evolving landscape of pneumococcal vaccination.

The top women's health stories in 2025 included groundbreaking advancements in women's health against an alarming mortality trend.

Paying for these new therapies, that may be curative for patients, remains a challenge.

The approval represents the first non-statin treatment indicated to lower low-density lipoprotein for primary prevention patients.

A clinical trial showed that nivolumab plus chemotherapy significantly improved overall survival and progression-free survival compared with a chemotherapy combination alone.

Eosinophilic esophagitis is a chronic, progressive disease thought to be responsible for damaging the esophagus, and it can severely impact a child’s ability to eat.

A Q&A with Kevin Malloy, PharmD, BCPS, on how diabetes technology has improved and how it can help pregnant people manage their disease.

A Q&A with Diana Isaacs, PharmD, BCPS, BCACP, BC-ADM, CDCES, FADCES, FCCP, director of education and training in diabetes technology at the Cleveland Clinic.

Sandoz's injection treatment natalizumab-sztn (Tyruko) is the first biosimilar approved by the health agency for the treatment of multiple sclerosis.
Researchers at the 2023 American Academy of Dermatology Association Annual Meeting discussed the effects of product complexity on preclinical testing, among other topics.

The approval was based on a confirmatory trial that found the therapy slowed disease progression compared with placebo.

Speakers at the 2023 virtual Value-Based Insurance Design Summit discussed government programs and company initiatives to advance health equity, as well as the role of research and advocacy to achieve pharmacoequity.

The new report reviewed the potential impact of biosimilars across multiple specialties.

Efanesoctocog alfa is a recombinant factor VIII therapy with once-a-week dosing.

The launch of Amgen's adalimumab biosimilar Amjevita was just the start of a wave of biosimilars to treat autoimmune diseases.

Sacituzumab govitecan significantly improved overall survival over the comparator chemotherapy in patients with hormone receptor–positive, human epidermal growth factor receptor 2–negative breast cancer who had received prior treatment.
The approval makes zanubrutinib available to all patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) whether they are newly diagnosed or previously treated.
A national study in Germany identified the baseline characteristics of patients with moderate-to-severe atopic dermatitis (AD) to understand how they are usually treated with Dupixent in the real world.

Patients with persistently controlled atopic dermatitis who had their doses reduced were able to maintain their low disease activity.
The increases had no impact on the efficacy of the drug and were rarely associated with symptoms or sequelae.

Certain problems related to health-related quality of life (HRQOL) became more common among patients with atopic dermatitis since the start of the COVID-19 pandemic.
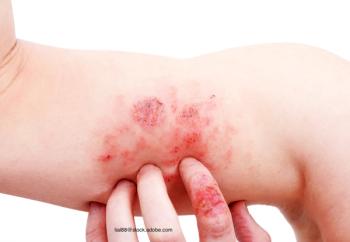
Data gathered from an electronic medical record found the results in a real-world patient population with atopic dermatitis were consistent with those found in clinical trials.

President Biden is reviving the program he headed as vice president, with the goal of reducing the cancer mortality rate and improving the experience of patients and their loved ones.

Published: February 8th 2022 | Updated:

Published: April 30th 2024 | Updated: