
With supply chain issues impacting many industries, drug shortages have occurred. How does the FDA address them?

With supply chain issues impacting many industries, drug shortages have occurred. How does the FDA address them?
Relyvrio is intended to slow the disease progression of amyotrophic lateral sclerosis.

Winner of SingleCare's Best of the Best: Best Pharmacy Technician, Santon joined Drug Topics® to discuss what pharmacy technicians can do for patients and why it's so important to her for every patient to afford their medication.

A group of pharmacists rose to the occasion to help a man having a medical emergency aboard a recent United flight bound for Newark, New Jersey.

Your weekly roundup of the latest news from Drug Topics®.

Mark Cuban’s Cost Plus Drugs business has entered into agreements with Capital Blue Cross and Rightway.

Seven of the 10 top selling drugs with high price increases lack the clinical evidence to justify those increases.
Pharmacists can optimize medication adherence and serve as an additional touchpoint for patients during treatment.

Short-term manufacturing problems are happening as the demand for Adderall and other stimulant medications for ADHD have increased. Data from Arrive Health show a marked increase in ADHD medication prescriptions for people in their 30s.

Your weekly roundup of the latest news from Drug Topics®.

Lecanemab is an investigational anti-amyloid beta antibody to treat mild cognitive impairment due to Alzheimer’s disease. It is currently under review by the FDA and a decision is expected by January 6, 2023.

The treatment is approved for preventing recurrent Clostridioides difficile infections.

A look at what’s to come in the December issue of Drug Topics®

Privacy rules to improve coordination of care, avoid discrimination in treatment.

Take a look back at our most popular news from November 2022.
The fight to put the consumer first in health care remains as many Americans feel the industry is still putting the mighty dollar ahead of patients.

Your weekly roundup of the latest news from Drug Topics®.

Take a look back at our most popular health system coverage for 2022.

Take a look back at our most popular COVID-19 coverage for 2022.

The reference to “maximum fair price” in the act bodes poorly for manufacturers and suggests more of a take-it-or-leave-it situation rather than a negotiation where clinical evidence would be the prevailing factor in determining price.
Take a look back at our most popular diabetes coverage for 2022.

CPESN payers and their success stories.

CSL Behring's gene therapy, called EntranaDez, may be approved later this month. BioMarin, Pfizer, Bayer and Freeline Therapeutics also have gene therapies for hemophilia in development.

Your weekly roundup of the latest news from Drug Topics®.

Health disparities in care were made worse by the pandemic.

FDA keeps its user fees but fails to gain important reforms.

Inflation is the top reason that nearly one-third of Americans are concerned about covering unexpected health care needs
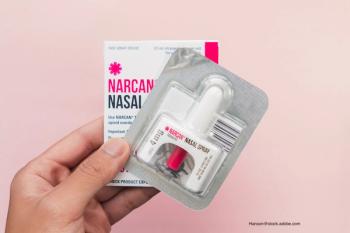
We may be one step closer to nonprescription naloxone.

Your weekly roundup of the latest news from Drug Topics®.
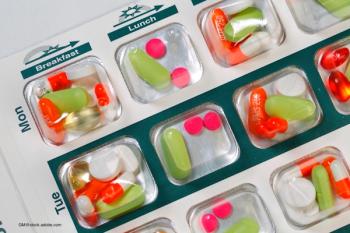
As a health plan worked to boost medication adherence among members, it made a startling discovery.