
In a virtual session held during the ASHP Midyear 2020 Clinical Meeting & Exhibition, experts discussed updates in asthma management, including new data and upcoming guideline changes that will impact pharmacy practice.

In a virtual session held during the ASHP Midyear 2020 Clinical Meeting & Exhibition, experts discussed updates in asthma management, including new data and upcoming guideline changes that will impact pharmacy practice.
A poster presented at the ASHP 2020 Midyear Clinical Meeting & Exhibition highlighted the success of an in-pharmacy pneumococcal immunization campaign.

Pharmacists may be among the first of the prioritized groups to be offered the COVID-19 vaccine once it receives FDA authorization.

Omalizumab (Xolair; Genentech and Novartis) is the first biologic approved for the treatment of nasal polyps that targets and blocks immunoglobulin E.
Interim analysis results from a phase 3 clinical trial reported a vaccine efficacy rate above 90% for BNT162b2. The trial is ongoing until it meets its threshold for confirmed COVID-19 cases.

With the accelerated use of digital health care amid the COVID-19 pandemic, investigators sought to provide evidence of its benefits for patients with COPD.

The UK study examined the cross-sectional association between vitamins A, E, C, and D and respiratory complaints.

Remdesivir (Veklury; Gilead) is the first and only fully FDA-approved treatment for COVID-19.

The study results indicate that clinicians should take cognitive dysfunction into account in the management of COPD.
CBD and its impacts on a protective peptide show that the cannabinoid may reduce lung inflammation and damage.

Results of a phase 3 trial showed clinical benefits with dupilumab (Dupixent; Sanofi and Regeneron) in children with uncontrolled moderate-to-severe asthma.
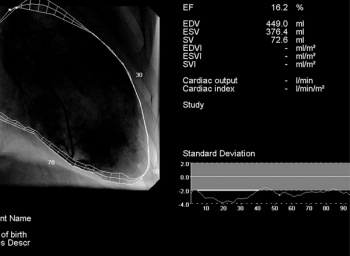
The meta-analysis accrued data from 18 studies that focused on patients with hospitalized and chronic heart failure.
In an open letter, Pfizer Chairman and CEO wrote that safety and efficacy data for its COVID-19 vaccine candidate may be ready by the third week of November for FDA submission.

An investigation examines whether an intervention including both school and home components improves asthma outcomes.

Help patients with upper respiratory infections choose an OTC product that’s right for them.
The BCG vaccine decreased the overall risk of new respiratory infections in elderly adults, according to the study results.

MJH Life Sciences™ COVID-19 Coalition will be hosting its next live webinar event “Navigating the COVID-19 Treatment Landscape: Clinical Controversies Explained” on Tuesday, October 13, 2020 at 6 PM EDT.
Investigators reported that, compared with chemotherapy, atezolizumab monotherapy was superior in overall survival and progression-free survival in patients with NSCLC.

Tocilizumab (Actemra; Genentech) reduced the likelihood of needing mechanical ventilation in hospitalized patients with COVID-19 associated pneumonia.

Perrigo has issued a voluntary recall of all unexpired albuterol sulfate inhalation aerosol to the retail level.

A new report illuminates the similarities and differences between COVID-19 and seasonal influenza.

Teva’s 2 digital inhalers for patients with asthma provide insight into objective inhaler use data.

Despite its popularity and its status as an FDA-approved expectorant, evidence to support guaifenesin’s efficacy is limited and conflicting.

Early data from a phase 3 trial showed that baricitinib combined with remdesivir improved recovery in patients hospitalized with COVID-19 compared with remdesivir alone.

Fluticasone furoate/umeclidinium/vilanterol (Trelegy Ellipta; GlaxoSmithKline) is indicated for the treatment of asthma in patients aged 18 years and older.