
Research being presented at CHEST 2024 analyzed morbidity and outcomes of children infected with influenza, COVID-19 or both.

Research being presented at CHEST 2024 analyzed morbidity and outcomes of children infected with influenza, COVID-19 or both.

The company will be presenting the full BATURA study data as a late-breaker oral presentation at the American College of Allergy, Asthma & Immunology (ACAAI) Annual Scientific Meeting.

This year’s meeting will take place from October 6 to 9 in Boston, Massachusetts.
Investigators suggested that the future cost-effectiveness of respiratory syncytial virus (RSV) vaccination could be improved through lower vaccine prices and longer-lasting protection.

The National Foundation for Infectious Diseases released a survey regarding the feelings of US adults toward influenza, COVID-19, RSV, and pneumococcal disease.

Dupixent is a fully human monoclonal antibody that inhibits the signaling of the IL4 and IL13 pathways.

Initiatives such as media campaigns and legislative rulings by the agencies have likely contributed to the decline.

A co-administration option for both vaccinations can increase the likelihood of older adults completing the vaccination series and improve health outcomes for the patient population.

Researchers analyzed the outcomes of positive airway pressure therapy for Medicare beneficiaries with obstructive sleep apnea.

Data from the SWIFT-1 and SWIFT-2 studies were presented by GSK at the European Respiratory Society International Conference.
The government said the tests will “detect current COVID-19 variants and can be used through the end of the year.”
Novavax is still awaiting FDA authorization for their protein-based COVID-19 vaccine.
Final approval of the therapy may occur after expiration of the 3-year regulatory exclusivity for Tyvaso DPI on May 23, 2025.

Ragweed season begins in late August and can run through early November.

The composition of the latest flu vaccine has changed. Here’s what pharmacists need to know.

The American College of Allergy, Asthma, and Immunology (ACAAI) recently released its 5 tips to help children and their parents stay educated on asthma peak month.

MK-1654 (clesrovimab) met its primary efficacy endpoint of incidence of patients with RSV-associated medically attended lower respiratory infections (MALRI) through Day 150.
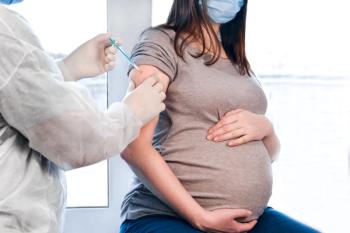
Current recommendations suggest women receive the RSV vaccine between 32 and 36 weeks' gestational age.
The updates follow the Advisory Committee for Immunization Practice meeting held June 26 to 28, 2024.

Test your pharmacy news knowledge with our weekly quiz.

The first-in-class selective dual PDE3 and PDE4 inhibitor from Verona Pharma is the first inhaled product with a novel mechanism of action approved to treat COPD in over 20 years.
The vaccine covers 8 serotypes not currently covered by any FDA-approved pneumococcal vaccines.

The new task force highlights a stronger government focus on curbing the risks of e-cigarettes for young people, especially after sustained calls for stricter action on the public health issue by lawmakers and the public.
The vaccine, mRNA-1083, elicited noninferior responses vs individual COVID-19 and flu vaccinations.

All Boehringer Ingelheim inhalers are included in the program and will be available for $35 per month for eligible patients.