ESMO released changes to its recommendations on how to manage patients with cancer during the COVID-19 pandemic.

ESMO released changes to its recommendations on how to manage patients with cancer during the COVID-19 pandemic.
A recent study evaluated the effects of the COVID-19 pandemic on normal cancer care activities, including cancer screening efforts.
On May 8, 2020, the FDA granted accelerated approval to selpercatinib (Retevmo; Eli Lilly and Company).

Karyopharm announced that the agency has accepted for filing its supplemental New Drug Application for selinexor as a second-line treatment for multiple myeloma.

A recent study found that the programs may result in cost savings to health systems and patients.

The fixed-dose combination of pertuzumab and trastuzumab with hyaluronidase (Phesgo; Genentech) can be administered subcutaneously for patients with HER2-positive breast cancer.

Today, pharmacists are banding together to “eliminate racism, discrimination, injustice, and the marginalization of individuals within the profession.”
An exploratory analysis of 2 phase 3 trials evaluating ribociclib (Kisqali, Novartis) plus endocrine therapy in advanced breast cancer showed positive results.

Oncology pharmacy practitioners highlight challenges amid the pandemic, such as limited access to personal protective equipment and essential medications.

The study, presented at the virtual ASCO20, showed significant clinical benefit for patients with endometrial cancer who were treated with cabozantinib and nivolumab.
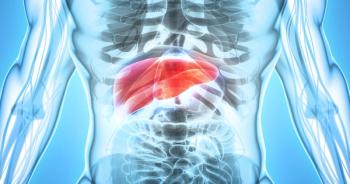
Officials with the FDA approved atezolizumab (Tecentriq, Genentech) in combination with bevacizumab (Avastin) for the treatment of patients with unresectable or metastatic hepatocellular carcinoma who have not received prior systemic therapy.

A systematic review of medical cannabis and cannabinoid use in oncology care demonstrated varied results.
Targeted therapy osimertinib (Tagrisso) demonstrated significant improvements in disease-free survival in patients with localized non-small cell lung cancer (NSCLC) who were treated following surgery.

The study marks the first time that the drug has demonstrated benefit for front-line therapy in patients with this type of colorectal cancer.

An outpatient multiple myeloma clinic benefited substantially from employing a clinical pharmacist.
“Data collection is ongoing with additional analyses planned to look at patient and provider perception of COVID-19 impact on cancer care.”
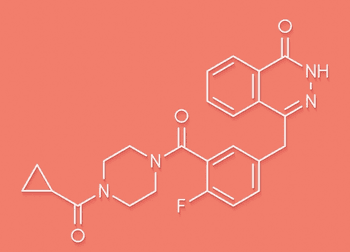
SOLO-2 is the first phase 3 trial to provide overall survival (OS) data on maintenance PARP inhibitor therapy.

The study is the first of its kind on a national scale to show the benefit of Medicaid expansion on cancer mortality rates.

The findings, which were presented virtually in the 2020 ASCO Virtual Scientific Program, included the association between treatment with hydroxychloroquine plus azithromycin and increased risk of death.

The American Society of Clinical Oncology (ASCO) recently released a detailed guidance to oncology practices

Approximately 20% to 30% of men with metastatic castration-resistant prostate cancer have HRR gene mutation.
Atezolizumab demonstrated a significant overall survival benefit in individuals with high PD-L1 expression compared with chemotherapy.
Officials with the FDA have granted breakthrough therapy designation to trastuzumab deruxtecan (Enhertu, AstraZeneca and Daiichi Sankyo) for the treatment of HER2-mutant metastatic non-small cell lung cancer (NSCLC).
Rucaparib is the first PARP inhibitor that has been approved in a prostate cancer setting.

Fam-trastuzumab deruxtecan-nxki (Enhertu, Daiichi Sankyo) is indicated for treating adults with unresectable or metastatic HER2-positive breast cancer who have received 2 or more prior anti-HER2–based regimens in the setting of metastasis.