
Fewer hospitalized patients with COVID-19 pneumonia died when treated with standard care plus either infliximab or abatacept.

Fewer hospitalized patients with COVID-19 pneumonia died when treated with standard care plus either infliximab or abatacept.
Heterologous vaccination—or mixing different vaccines—was safe and effective with Novavax and provided enhanced protection against the Omicron variant, a new study found.
Pending authorization of the XBB.1.5 vaccine, the company said that doses would be available in time for fall vaccination.
In a large observational study, significant information was obtained about mRNA vaccine protection against acute infection and disease severity.
The VRBPAC committee voted 21-0 in favor of this recommendation.

The newly approved at-home test was effective in trials, correctly identifying 98.7% of negative and 92.9% of positive samples for individuals with symptoms of upper respiratory infection.

Patients were more likely to require ICU admission.

New research suggests that chronotropic incompetence contributes to exercise limitations in Long COVID.
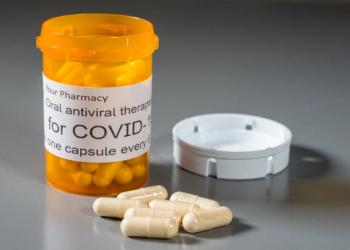
More than 11.6 million treatment courses of the medication have been prescribed in the U.S. to date.

Finalizing a definition for postacute sequelae of SARS-CoV-2 infection will lead to high-quality care and treatment for patients suffering from the condition.

Quality of life (QOL) for people living with autoimmune diseases benefits from an integrated approach that considers individual immune system sensitivities and patients’ life circumstances, a new study shows.

New data from Digestive Disease Week furthers evidence that biologics can be effective

Data presented at Digestive Disease Week show that patients with IBD had less intense post-vaccination symptoms after a fourth dose.

According to commentaries published in the Annals of Internal Medicine, doctors remain split on the question.
How much progress have we made in developing therapies to treat this common childhood respiratory disorder?

Following some improvement, excess death rate returned to 1999 levels for Black men and 2005 levels for Black women.
Unvaccinated patients who were older at MG onset and at SARS-CoV-2 infection had more severe cases.

While acknowledging remaining uncertainties posed by potential evolution of SARS-CoV-2, the organization advised it’s time to transition to long-term management of the pandemic.

Though the Omicron variant caused the most symptoms in pediatric patients, there were no differences in adverse outcomes by COVID-19 variant.

According to a recent study, pregnant women infected with COVID-19 experienced significant maternal mortality during pregnancy, with worse outcomes during the second wave of the pandemic compared to the first.
Monovalent vaccines by these manufacturers are no longer authorized for use in the United States.

Despite a massive COVID-19 vaccination campaign, many still fear a return to normal.
Project NextGen will focus on the development of vaccines and long-lasting monoclonal antibody COVID-19 treatments.

Researchers of a new study investigated what's causing vaccine hesitancy, and why individuals are feeling vaccine fatigue.
The FDA granted the EUA based on the phase 3 trial results that demonstrated vilobelimab's efficacy compared to placebo, with a 23.9% relative reduction in all-cause mortality from COVID-19 at 28 days.