Shingles Vaccine Remains Important for Adults 50+
The CDC recommends Shingrix for prevention of herpes zoster
Amidst the ongoing focus on getting COVID-19 vaccines into arms and seasonal reminders about the flu vaccine, it’s easy to forget that there are other important vaccines that adults should receive. That’s why it’s especially critical for pharmacists and other healthcare providers to be proactive about keeping their patients up to date when it comes to the CDC’s Recommended Adult Immunization Schedule.1
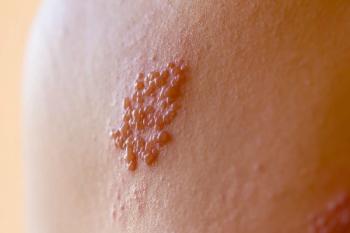
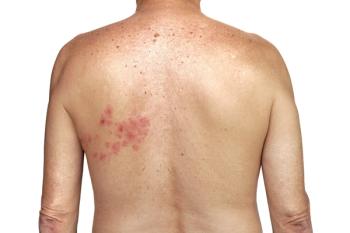
Included on that list is vaccination against herpes zoster (shingles) and its related complications. Shingrix, the recombinant zoster vaccine, is the only shingles vaccine available in the United States, and is recommended for immunocompetent adults aged 50 and older. It is estimated that virtually all individuals in this demographic have been exposed to the varicella zoster virus (VZV), which causes both chickenpox (varicella zoster) and shingles.
According to the CDC, approximately 1 out of 3 individuals in the United States will develop shingles in their lifetime, a
Shingrix is administered in 2 doses delivered 2 to 6 months apart. Pharmacists and other providers do not need to screen for a history of chickenpox infection or test for serologic evidence of prior infection before administering the vaccine.
Under CDC guidelines, individuals can be vaccinated with Shingrix whether or not they report a prior episode of shingles, or prior vaccination with Zostavax.3
“There is no specific amount of time you need to wait before administering Shingrix to patients who have had herpes zoster,” the CDC wrote on its website. “However, you should not give Shingrix to patients who are experiencing an acute episode of herpes zoster.”4 In such cases, vaccination should be postponed until the acute stage of the illness has passed and the patient is no longer symptomatic.
Patients with chronic medical conditions like diabetes, rheumatoid arthritis, and pulmonary disease can be vaccinated. Likewise, patients who are taking low-dose immunosuppressive therapy, are anticipating immunosuppression, or have recovered from an immunocompromising illness are also eligible.
Additionally, Shingrix may be administered concurrently with other vaccines on the CDC’s Recommended Adult Immunization Schedule. These include
There are a few contraindications for herpes zoster vaccination. Shingrix should not be administered to a person who had an allergic reaction after a previous dose of the vaccine or has a history of severe allergic reaction to any component of the vaccine. If a person is known to be seronegative for varicella, providers should follow the CDC’s Advisory Committee on Immunization Practices (ACIP)
As ACIP notes, Shingrix’s
“With high efficacy among adults aged ≥50 years, and modest waning of protection over 4 years following vaccination, RZV has the potential to prevent substantial herpes zoster disease burden,” ACIP guidance concluded.5 “Vaccinating adults starting at age 50 will prevent disease incidence in midlife, and the vaccine will likely continue to provide substantial protection beyond 4 years as recipients age.”
References
- Recommended adult immunization schedule for ages 19 years or older, United States, 2021. CDC. Updated February 12, 2021. Accessed November 24, 2021.
https://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html - Shingles burden and trends. CDC. Updated August 14, 2019. Accessed November 24, 2021.
https://www.cdc.gov/shingles/surveillance.html - Shingles vaccination. CDC. Updated January 25, 2018. Accessed November 24, 2021.
https://www.cdc.gov/vaccines/vpd/shingles/public/shingrix/index.html - Shingrix recommendations. CDC. Updated October 5, 2020. Accessed November 24, 2021.
https://www.cdc.gov/vaccines/vpd/shingles/hcp/shingrix/recommendations.html - Dooling KL, Guo A, Patel M, et al.
Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. Morb Mortal Weekly Rep. 2018;67(3):103-108. doi:10.15585/mmwr.mm6703a5
Newsletter
Pharmacy practice is always changing. Stay ahead of the curve with the Drug Topics newsletter and get the latest drug information, industry trends, and patient care tips.