- Drug Topics September 2023
- Volume 167
- Issue 08
Assessing and Treating Opioid Use Disorder in Pregnancy
Opioid use disorder during pregnancy is stigmatized, but these patients deserve compassionate, nonjudgmental care.
Opioid use and opioid use disorder (OUD) have profoundly impacted the United States over the past 2 decades. The opioid crisis began with the overprescribing of prescription opioids and has had distinct subsequent waves, first with heroin and more recently with synthetic opioids, including illicitly manufactured fentanyl.1-3 Overdose rates have continued to rise, with more than 100,000 individuals dying from overdose in 2022.4
The ages associated with the highest prevalence of substance use and development of use disorder overlap, especially during the earlier years of pregnancy potential. Hence, opioid use, OUD, and overdose in pregnancy and postpartum have increased in parallel with the opioid crisis, making overdose a leading cause of pregnancy-associated deaths.2
Most adults in the United States have used substances; however, only a minority of people develop a use disorder.5 Risk factors for development of substance use disorder include a younger age of first use, substance availability in both the household and the neighborhood, preexisting mental illness, and—especially for women— gender-based violence, including childhood sexual abuse, that leads to trauma.6,7
Although medications for OUD (MOUDs) have been available for more than 50 years, most pregnant women with OUD receive no treatment because of inadequate access, ambivalence, and fear of stigma and discrimination.8-10 By integrating OUD assessment and treatment into prenatal and postpartum care, health care providers can directly address the opioid crisis and interrupt the generational cycle of addiction.11
Stigma and Language
Stigma is the process by which people are “marked” by a perceived differentness of deviation from social norms.12 Stigma is often expressed in language and experienced by people as discrimination. A large body of evidence documents how common stigmatizing beliefs are among health care providers and how the experience of health care discrimination negatively impacts care.13-17 Pregnant women who use drugs experience compounded stigmas (of substance use, pregnancy, treatment) and consequentially may be legitimately fearful to disclose their use despite desiring treatment.
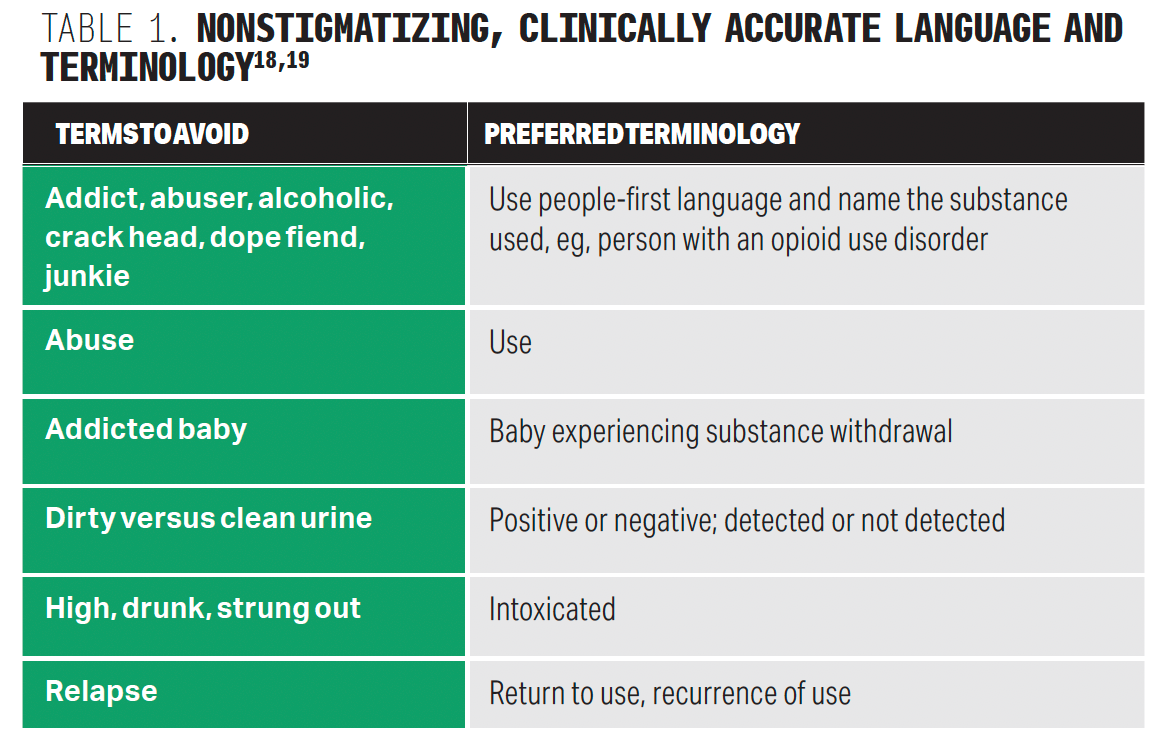
Responsibility of ensuring patient safety rests upon the provider. It is important to that providers interact with patients in a nonjudgmental way and provide support to patients who are at a variety of entry points of change (Table 1).18,19 Harm reduction should be explored, including naloxone and syringe services and safer injection practices if appropriate.20
Assessment
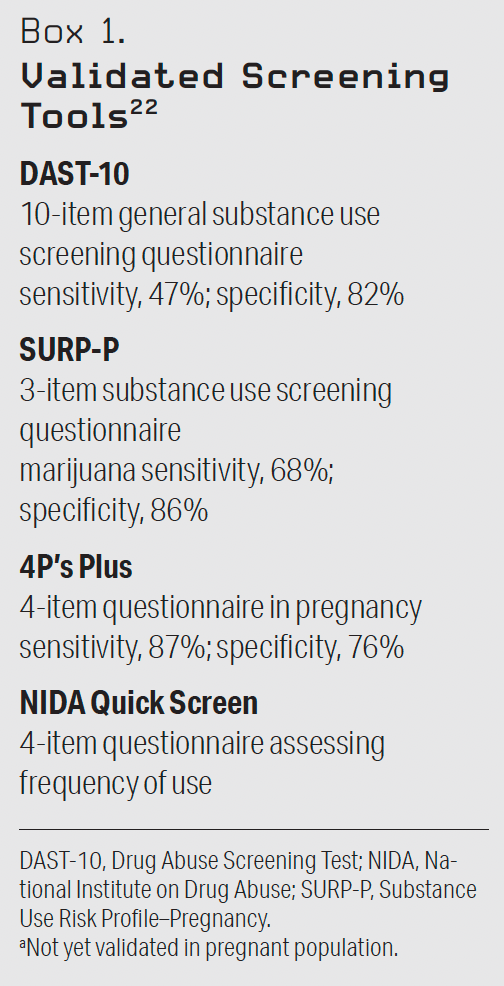
Screening is the primary means by which substance use is identified clinically, whether the patient is pregnant or not.21 Screening should be distinguished from testing. Screening is characterized by a series of questions or use of a validated tool, such as a questionnaire.21 See Box 1 for examples of validated screening tools.22 Testing is the examination of a biological specimen (such as urine) for the presence of drug metabolites or parent compounds. Although universal screening is recommended for all pregnant women by the American College of Obstetricians and Gynecologists, drug testing is not.23
“Presumptive tests” utilize ELISA technology and are the most common testing used. Although they render results rapidly, providers should be aware that the information quality obtained from a presumptive test is poor because of a high rate of both false-positive and false-negative results. Definitive tests use the technology of liquid or gas chromatography–mass spectrometry and provide quantified results and exact substance identification.24 Definitive tests are more expensive and take days to report. In addition, the window of detection varies by substances and for many is typically fewer than 3 days.25 The primary symptoms of addiction are behaviors, and a positive drug test cannot identify a substance use disorder, much less parenting competence, even when a positive result is confirmed.21 Finally, professional society recommendations are unanimous: Drug testing, if done, requires informed written patient consent.
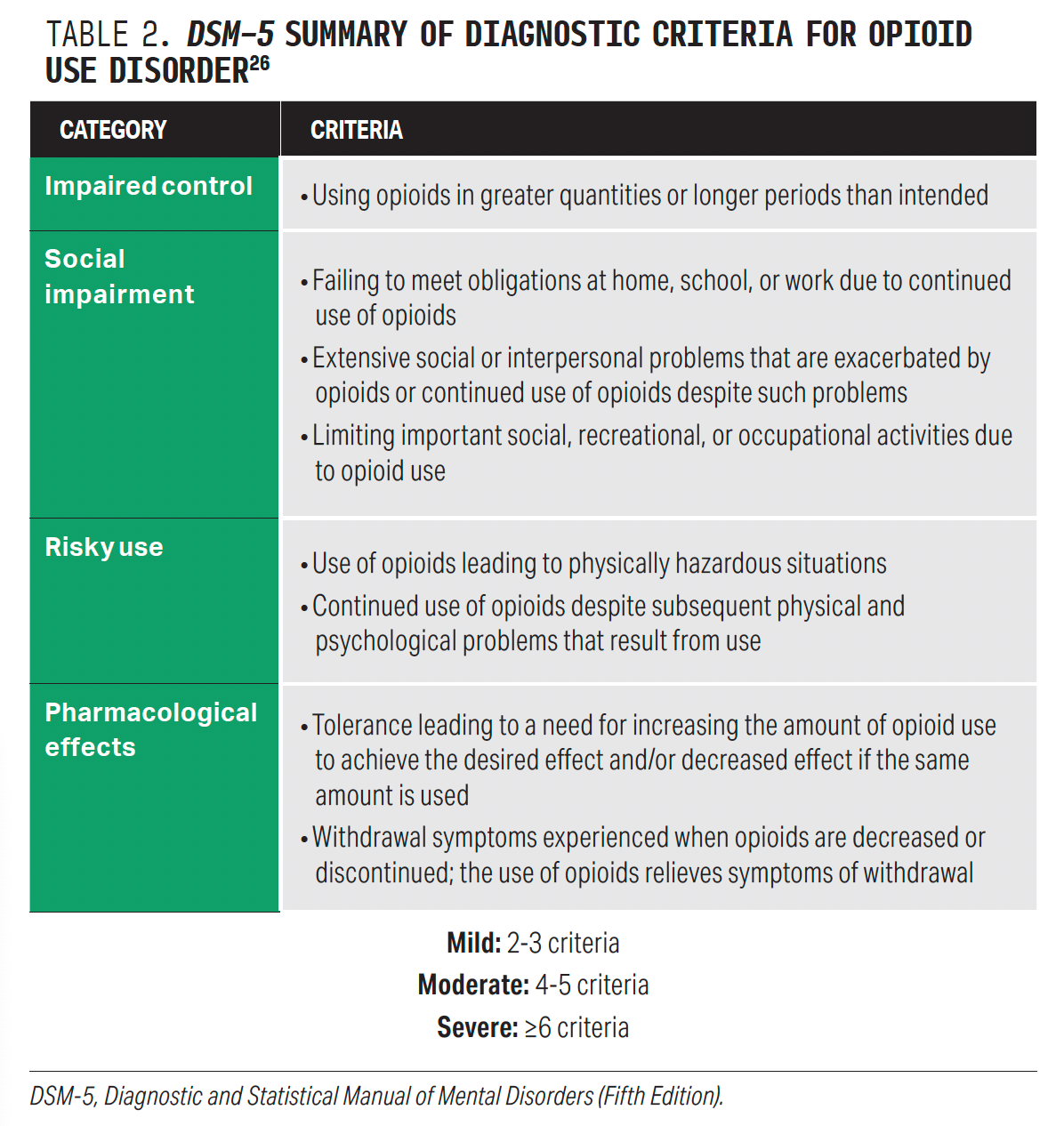
Assessment for substance use should be performed via validated screening instrument. A positive screen should be followed by diagnostic assessment. The Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition) diagnostic criteria should be used to diagnose OUD (Table 2).26 To meet criteria for OUD, 2 or more affirmative responses to the criteria are required.27
Treatment
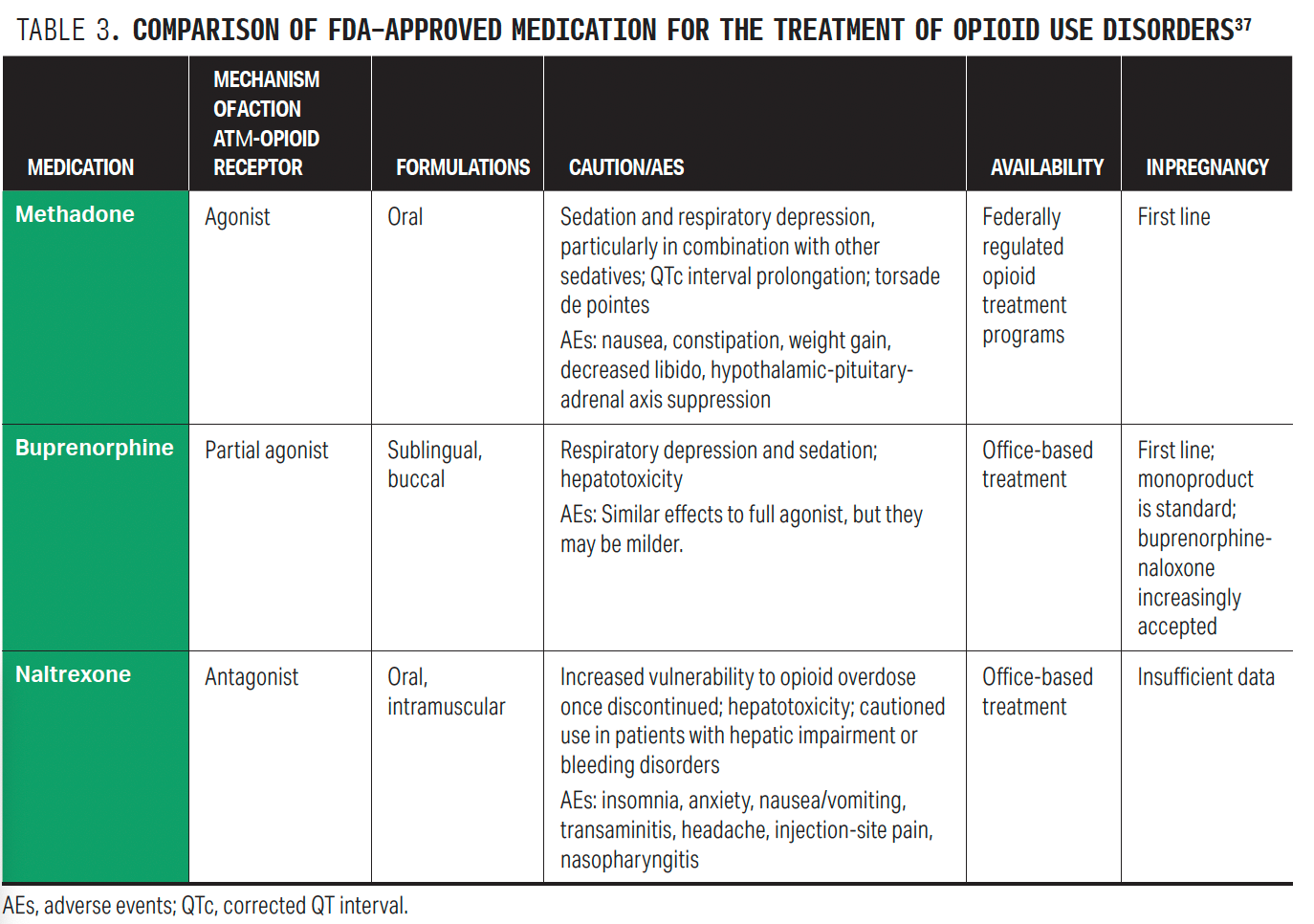
After confirmation of OUD, treatment should be offered. The standard of care for OUD rests on medications. Methadone and buprenorphine are the safest and most effective MOUDs in pregnancy; there are less data for naltrexone in pregnancy.
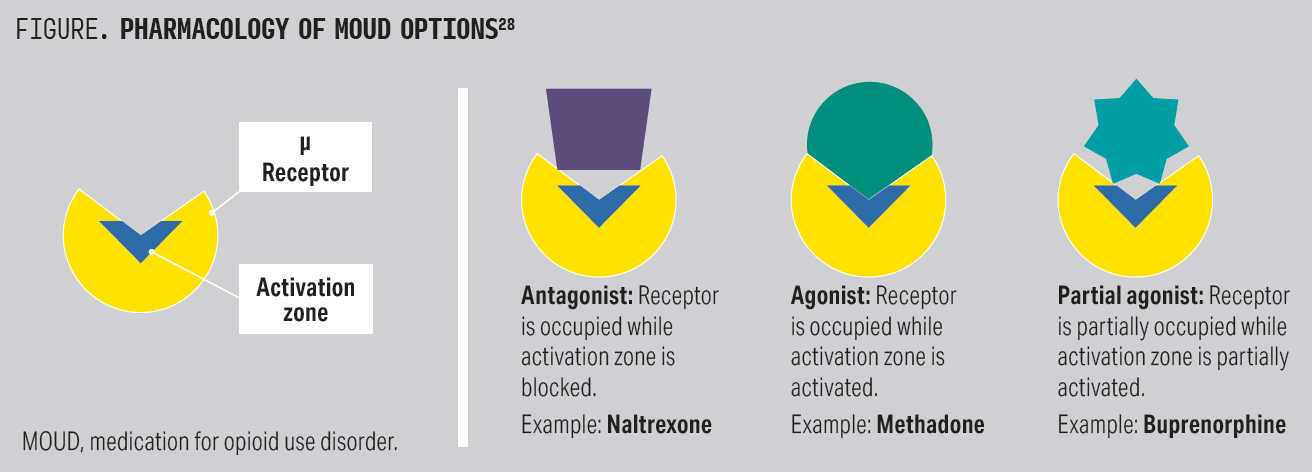
These 3 medications differ in pharmacology: Methadone is a full opioid receptor agonist, buprenorphine is a partial agonist, and naltrexone is an opioid antagonist (Figure).28 The μ-opioid receptor is the primary site of action and is responsible for many of the favorable aspects of opioids, namely euphoria, analgesia, and stress coping. However, MOUD also exerts activity on both δ- and κ-opioid receptors, which are important to understand in terms of potential medication adverse effects. δ-Receptor activation leads to anxiolysis and positive affect, whereas κ activation is associated with dysphoria, stress, and negative affect.29 Methadone is a full agonist at the μ, δ, and κ receptors; buprenorphine is a partial μ agonist and a δ and κ antagonist; and naltrexone is an antagonist at the μ, δ, and κ receptors.30
Opioids are addictive, in part because they are both positively and negatively reinforcing. These dual characteristics lead to neuroadaptations that follow chronic use, causing a patient to develop tolerance, experience withdrawal when the substance is not used, and experience cravings. MOUD works first by treating opioid withdrawal and then by controlling drug cravings, which interrupts negative reinforcement. MOUD also works by establishing an “opioid blockade” (blocking the effect of other opioids if used), which interrupts positive reinforcement and establishes a cross-tolerance. Collectively, these features are associated with decreased mortality and reduced substance use.31
Behavioral therapy can be an important dimension of recovery for many people. However, the overall literature on OUD treatment demonstrates little to no additional benefit of behavioral therapies such as group counseling vs medication. Therefore, providers should support behavioral therapy but not insist upon it—and certainly not withhold medication for counseling nonadherence. Some patients may be reluctant or resistant to initiate MOUD because of prior negative experiences with medication or because of mistrust or family/peer pressure. Engagement in prenatal care, even in the context of continued substance use, improves birth outcomes, and providers should utilize a harm reduction framework, in which the provider not only “meets the patient where they are,” but also “helps them get where they want to go.”32
MOUDs
Methadone is among the most federally regulated medications in the United States; it cannot be prescribed for OUD but can be administered directly to patients in the outpatient setting from a federally licensed opioid treatment program (OTP). Methadone can also be initiated or continued in a hospital. Methadone does not require withdrawal for initiation.33 Because of its differential metabolism between individuals, initiation is often slower than for buprenorphine. However, pregnancy is a rapid metabolic state, and more rapid protocols have been suggested.34
Buprenorphine is less regulated and, hence, more easily integrated into prenatal care settings. The Drug Enforcement Administration X-waiver, which had previously limited who could prescribe buprenorphine, was recently removed. Buprenorphine can now be prescribed by any provider with prescribing authority.35 This regulatory change should increase access to buprenorphine both for medication initiation and continuation.
Buprenorphine is a partial agonist: The patient needs to be withdrawn before medication initiation. If not, buprenorphine can precipitate withdrawal.33 Although there is concern that fentanyl might lead to a greater likelihood of precipitated withdrawal, population health data to support this are lacking, and the likelihood of precipitated withdrawal can be lessened with microdosing or cross-tapering protocols. Buprenorphine is available in 2 formulations: sublingual films or tablets and a monthly extended release.33 Although the initial literature recommended the use of the buprenorphine monoproduct in pregnancy, subsequent research has concluded that the combination product of buprenorphine plus naloxone is as safe and effective.36
Naltrexone is an opioid receptor antagonist and requires a week of opioid abstinence prior to initiation to avoid precipitated withdrawal. Naltrexone can be prescribed by anyone with prescribing authority and comes in both oral and extended-release formulations.33 The existing literature on naltrexone in pregnancy is limited to case series. There are less data on the safety and effectiveness of naltrexone compared with buprenorphine or meth adone, so its use is not recommended.24 See Table 3 for a comparison of medications.37
Opioid Withdrawal and Buprenorphine Initiation
Withdrawal is the inverse of dependence and occurs following cessation of opioid use or administration of either a partial or a full opioid antagonist. Chronic opioid use leads to dependence on sympathetic and parasympathetic control by the exogenous opioid, and withdrawal, consequentially, leads to noradrenergic imbalance seen in the increase of norepinephrine from the locus coeruleus, which leads to the typical symptoms of withdrawal: irritability, abdominal cramps, nausea/vomiting, diarrhea, increased blood pressure and pulse rate, lacrimation, rhinorrhea, yawning, and pupil dilation.34 Withdrawal can be clinically measured using the Clinical Opiate Withdrawal Scale and should be assessed at every visit, especially early in treatment and in pregnancy.
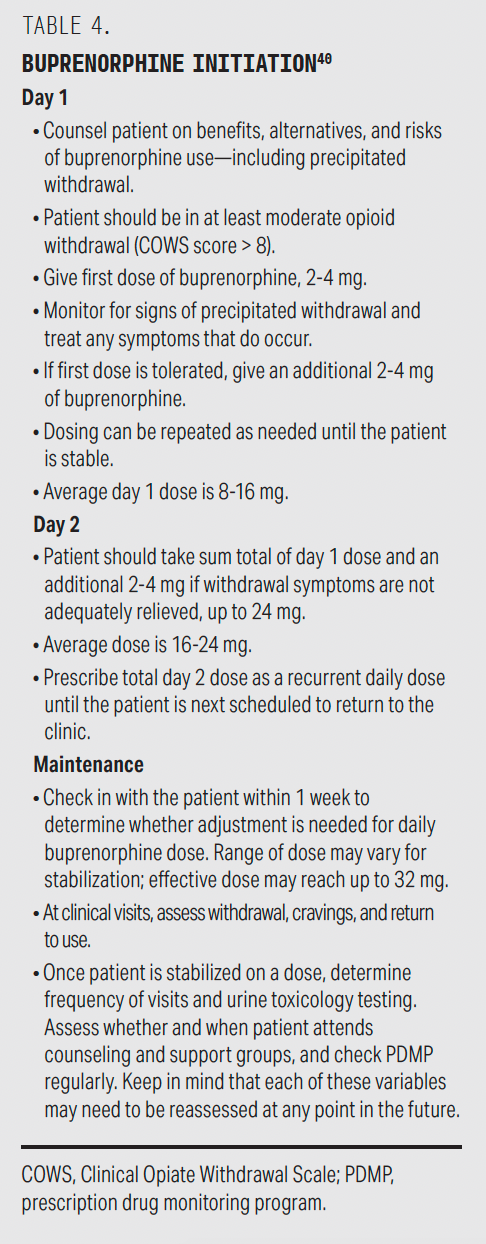
Because buprenorphine is a partial agonist, withdrawal may be precipitated if the medication is initiated too early. Standard buprenorphine initiation protocols begin medication administration when the Clinical Opiate Withdrawal Scale score is at least 8.38 However, microdosing or cross-tapering protocols can be started before the patient has experienced withdrawal.39 These protocols begin with low-dose buprenorphine administration (typically 0.5 mg), below the threshold of precipitated withdrawal (Box 2). Table 4 describes a standard buprenorphine initiation protocol.40
MOUD Care Continuity
Following stabilization on MOUD, patients should be assessed for withdrawal, cravings, and other substance use. Both metabolic and physiologic changes of pregnancy can lead to more rapid metabolism of both methadone and buprenorphine. Hence, some patients who had previously been stable on a medication dose may present with increased drug cravings or even withdrawal symptoms.
In this circumstance, providers should consider split dosing (twice, 3 times, or even 4 times daily) before dose increases, especially if the symptoms occur hours after medication administration. Split dosing increases the pharmacokinetic area under the curve by minimizing peaks and troughs and encourages a more optimal steady state. For methadone, split dosing provided by the OTP in the form of take-home bottles should be considered and fully discussed with the patient.24
The Importance of Naloxone
Naloxone is a short-acting opioid antagonist with high affinity for the μ-opioid receptor. It is highly effective in reversing an overdose through the displacement of other opioids at the μ receptor. Anyone who uses opioids (prescribed, MOUD, or untreated OUD) and anyone who may witness an overdose (such as friends and families of people who use opioids) should receive naloxone and know how to use it.41 This is particularly important postpartum because overdose is a leading cause of maternal death in the United States and timely administration of naloxone saves lives.2
Naloxone can be prescribed, distributed directly often through local public health agencies, or obtained from a pharmacy under a regional standing order. The FDA recently approved naloxone for OTC sale. Naloxone has a short half-life, and its effect begins to wear off within 10 minutes. People can be reversed with naloxone and then slip back into overdose again. Therefore, multiple doses are often needed, and patients and families should be advised to call 911 following an overdose event.
Conclusion
Health care providers are well positioned to assess, diagnose, and initiate treatment for OUD in pregnancy and through the postpartum period. Methadone and buprenorphine are the safest and most effective medications for OUD in pregnancy. Utilizing the key fundamental principles of OUD management, providers will be better prepared to confidently introduce this lifesaving treatment into their practice repertoire.
Leah Habersham, MD, MBA, MS, is an assistant professor in the Departmentof Psychiatry and the Department of Obstetrics, Gynecology, and Reproductive Science at Icahn School of Medicine at Mount Sinai.
Mishka Terplan, MD, MPH, is the medical director at Friends Research Institute and adjunct faculty in the Department of Family and Community Medicine at University of California, San Francisco.
References
Dayer LE, Painter JT, McCain K, King J, Cullen J, Foster HR. A recent history of opioid use in the US: three decades of change. Subst Use Misuse. 2019;54(2):331-339. doi:10.1080/10826084.2018.1517175
Bruzelius E, Martins SS. US trends in drug overdose mortality among pregnant and postpartum persons, 2017-2020. JAMA. 2022;328(21):2159-2161. doi:10.1001/jama.2022.17045
Understand the opioid overdose epidemic. CDC. Updated June 1, 2022. Accessed June 22, 2023.
https://www.cdc.gov/opioids/basics/epidemic.html Ahmad FB, Cisewski JA, Rossen LM, Sutton P. Provisional drug overdose death counts. National Center for Health Statistics. 2023. Accessed June 22, 2023.
https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm Emerson B, Haden M, Kendall P, Mathias R, Parker R. A public health approach to drug control in Canada. Health Officers Council of British Columbia. October 2005. Accessed June 22, 2023.
http://www.cfdp.ca/bchoc.pdf Cohen LR, Hien DA. Treatment outcomes for women with substance abuse and PTSD who have experienced complex trauma. Psychiatr Serv. 2006;57(1):100-106. doi:10.1176/appi.ps.57.1.100
Martini F, Fregna L, Bosia M, Perrozzi G, Cavallaro R. Substance-related disorders. In: Cavallaro R, Colombo C, eds. Fundamentals of Psychiatry for Health Care Professionals. Springer International Publishing; 2022:263-295.
Bedrick BS, O’Donnell C, Marx CM, et al. Barriers to accessing opioid agonist therapy in pregnancy. Am J Obstet Gynecol MFM. 2020;2(4):100225. doi:10.1016/j.ajogmf.2020.100225
Guille C, McCauley JL, Moreland A. Leveraging telehealth in the United States to increase access to opioid use disorder treatment in pregnancy and postpartum during the COVID-19 pandemic. Am J Psychiatry. 2021;178(4):290-293. doi:10.1176/appi.ajp.2020.20060949
Patrick SW, Richards MR, Dupont WD, et al. Association of pregnancy and insurance status with treatment access for opioid use disorder. JAMA Netw Open. 2020;3(8):e2013456. doi:10.1001/jamanetworkopen.2020.13456
Habersham LL. An unexpected path to addiction medicine. Am J Obstet Gynecol MFM. 2023;5(3):100849. doi:10.1016/j.ajogmf.2022.100849
Holder SM, Peterson ER, Stephens R, Crandall LA. Stigma in mental health at the macro and micro levels: implications for mental health consumers and professionals. Community Ment Health J. 2019;55(3):369-374. doi:10.1007/s10597-018-0308-y
Drabish K, Theeke LA. Health impact of stigma, discrimination, prejudice, and bias experienced by transgender people: a systematic review of quantitative studies. Issues Ment Health Nurs. 2022;43(2):111-118. doi:10.1080/01612840.2021.1961330
da Silva AG, Baldaçara L, Cavalcante DA, Fasanella NA, Palha AP. The impact of mental illness stigma on psychiatric emergencies. Front Psychiatry. 2020;11:573. doi:10.3389/fpsyt.2020.00573
Yuvaraj A, Mahendra VS, Chakrapani V, et al. HIV and stigma in the healthcare setting. Oral Dis. 2020;26(suppl 1):103-111. doi:10.1111/odi.13585
Calabrese SK. Understanding, contextualizing, and addressing PrEP stigma to enhance PrEP implementation. Curr HIV/AIDS Rep. 2020;17(6):579-588. doi:10.1007/s11904-020-00533-y
Wogen J, Restrepo MT. Human rights, stigma, and substance use. Health Hum Rights. 2020;22(1):51-60.
Raihan N, Cogburn M. Stages of Change Theory. StatPearls Publishing; 2023. Accessed July 17, 2023.
https://www.ncbi.nlm.nih.gov/books/NBK556005/ Ries R, Miller SC, Saitz R, Fiellin DA, American Society of Addiction Medicine, eds. The ASAM Principles of Addiction Medicine. Fifth edition. Wolters Kluwer Health/Lippincott Williams & Wilkins; 2014.
Strang J, Volkow ND, Degenhardt L, et al. Opioid use disorder. Nat Rev Dis Primers. 2020;6(1):3. doi:10.1038/s41572-019-0137-5
Ecker J, Abuhamad A, Hill W, et al. Substance use disorders in pregnancy: clinical, ethical, and research imperatives of the opioid epidemic: a report of a joint workshop of the Society for Maternal-Fetal Medicine, American College of Obstetricians and Gynecologists, and American Society of Addiction Medicine. Am J Obstet Gynecol. 2019;221(1):B5-B28. doi:10.1016/j.ajog.2019.03.022
Media BKK. Publications & guidelines | smfm. Org - the society for maternal-fetal medicine. Accessed July 18, 2023. https://www.smfm.org/publications/275-smfm-special-report-substance-use-disorders-in-pregnancy-clinical-ethical-and-research-imperatives-of-the-opioid-epidemic
American College of Obstetricians and Gynecologists. ACOG Committee Opinion, Number 711. Opioid use and opioid use disorder in pregnancy. ACOG. 2021. Accessed June 1, 2023. https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2017/08/opioid-use-and-opioid-use-disorder-in-pregnancy
Kale N. Urine drug tests: ordering and interpreting results. Am Fam Physician. 2019;99(1):33-39.
Moeller KE, Kissack JC, Atayee RS, Lee KC. Clinical interpretation of urine drug tests: what clinicians need to know about urine drug screens. Mayo Clin Proc. 2017;92(5):774-796. doi:10.1016/j.mayocp.2016.12.007
Brezing C, Bisaga A. Opioid use disorder: update on diagnosis and treatment. Psychiatric Times. Published online April 1, 2015. Accessed July 17, 2023. https://www.psychiatrictimes.com/view/opioid-use-disorder-update-diagnosis-and-treatment
Cleary EM, Smid MC, Charles JE, et al. Buprenorphine X-waiver exemption - beyond the basics for the obstetrical provider. Am J Obstet Gynecol MFM. 2021;3(6):100451. doi:10.1016/j.ajogmf.2021.100451
Valentino RJ, Volkow ND. Untangling the complexity of opioid receptor function. Neuropsychopharmacology. 2018;43(13):2514-2520. doi:10.1038/s41386-018-0225-3
Soyka M. Transition from full mu opioid agonists to buprenorphine in opioid dependent patients-a critical review. Front Pharmacol. 2021;12:718811. doi:10.3389/fphar.2021.718811
Ries RK, Fiellin DA, Miller SC, Saitz R eds. The ASAM Principles of Addiction Medicine. 5th ed. Lippincott Williams & Wilkins; 2014.
Ellis LP, Parlier-Ahmad AB, Scheikl M, Martin CE. An integrated care model for pregnant and postpartum individuals receiving medication for opioid use disorder. J Addict Med. 2023;17(2):131-139. doi:10.1097/ADM.0000000000001052
Taylor JL, Samet JH. Opioid use disorder. Ann Intern Med. 2022;175(1):ITC1-ITC16. doi:10.7326/AITC202201180
Iyer N, Ferguson E, Yan V, Hand D, Abatemarco D, Boelig RC. Comparing two protocols for inpatient methadone titration in pregnant patients with opioid use disorder. Am J Obstet Gynecol. 2023;228(1):S208. doi:https://doi.org/10.1016/j.ajog.2022.11.387
Knopf A. DEA supports removal of buprenorphine regulations. Alcoholism & Drug Abuse Weekly. 2023;35(4):5. doi:10.1002/adaw.33671
Ordean A, Tubman-Broeren M. Safety and efficacy of buprenorphine-naloxone in pregnancy: a systematic review of the literature. Pathophysiology. 2023;30(1):27-36. doi:10.3390/pathophysiology30010004
Srivastava AB, Mariani JJ, Levin FR. New directions in the treatment of opioid withdrawal. Lancet. 2020;395(10241):1938-1948. doi:10.1016/S0140-6736(20)30852-7
Taylor JL, Samet JH. Opioid Use Disorder. Ann Intern Med. 2022;175(1):ITC1-ITC16. doi:10.7326/AITC202201180
Moe J, O’Sullivan F, Hohl CM, et al. Short communication: systematic review on effectiveness of micro-induction approaches to buprenorphine initiation. Addict Behav. 2021;114:106740. doi:10.1016/j.addbeh.2020.106740
Jarvis M, Williams J, Hurford M, et al. Appropriate use of drug testing in clinical addiction medicine. J Addict Med. 2017;11(3):163-173. doi:10.1097/ADM.0000000000000323
Buprenorphine quick start guide. Substance Abuse and Mental Health Services Administration. Accessed June 1, 2023.https://www.samhsa.gov/sites/default/files/quick-start-guide.pdf
Duska M, Goodman D. Implementation of a prenatal naloxone distribution program to decrease maternal mortality from opioid overdose. Matern Child Health J. 2022;26(5):985-993. doi:10.1007/s10995-021-03307-z
Articles in this issue
over 2 years ago
The Role of Complementary and Alternative Medicine in Pharmacyover 2 years ago
Dance With the Girl That Brung Yaover 2 years ago
Review of FDA Approvals for Pediatric Obesity Managementover 2 years ago
Gut Health Has Impact on the Skinover 2 years ago
Biologics Offer Great Results in Treating Psoriasis—At a Costover 2 years ago
In A Post-Dobbs World, Pharmacists Still Face Uncertaintyover 2 years ago
How AI Can Improve Controlled Substance SecurityNewsletter
Pharmacy practice is always changing. Stay ahead of the curve with the Drug Topics newsletter and get the latest drug information, industry trends, and patient care tips.