
Selarsdi is interchangeable for all indications of ustekinumab, including treatment of psoriatic arthritis, plaque psoriasis, Crohn disease, and ulcerative colitis.

Selarsdi is interchangeable for all indications of ustekinumab, including treatment of psoriatic arthritis, plaque psoriasis, Crohn disease, and ulcerative colitis.

Type 1 diabetes can cause long-term complications, including the development of autoimmune diseases.
Previously, dupilumab (Dupixent) received orphan drug designation for bullous pemphigoid.
ADI-001 is an investigation allogeneic gamma delta chimeric antigen receptor T cell therapy that targets CD20 for the treatment of a variety of autoimmune diseases.

Despite an interest in services, a majority of women do not have these critical conversations with their health care providers.

Systemic lupus erythematosus is associated with a higher risk of cervical dysplasia due to the inflammatory nature of the disease.

Premenstrual Dysphoric Disorder is considered a psychiatric disorder and is associated with moderate to severe physical, affective, and behavioral symptoms.

The approval is supported by results from phase 3 multi-regional clinical trials conducted in patients with plaque psoriasis.
Genentech said it’s sharing the data with global health authorities to make the therapy available to patients as soon as possible.

Results from HERCULES will form the basis for future discussions with global regulatory authorities, according to Sanofi.

The initiative by Boehringer and GoodRx has the potential to accelerate the therapeutic timeline for patients treated with the biosimilar.

This approval is the second biosimilar approval for manufacturer Alvotech.

Due to the increased possibility of adverse pregnancy outcomes associated with methotrexate and leflunomid, it is crucial that patients with rheumatoid arthritis (RA) engage in thorough consultations with their physicians regarding pregnancy planning.

The biosimilar represents a comprehensive, accessible, and high-quality treatment option for patients in the US treated with tocilizumab.

Simlandi is the only biosimilar to Humira on the market that is high-concentration, citrate-free, and interchangeable.
Study authors theorized that the reduced effect could be due to disease-related or immunosuppressive treatment factors.

In addition to the numerous adalimumab (Humira) biosimilars that launched this year, multiple new biosimilar products were approved by the FDA in 2023.

Most patients were not concerned about losing control over their disease after switching.

Authors of a platinum-ranked poster presented at AMCP Nexus 2023 suggested that the burden of increased spending on specialty medications varies by race and wage among employees with employer-sponsored health insurance.

Newly approved by the FDA, Celltrion’s infliximab-dyyb (Zymfentra) offers patients with inflammatory bowel disease a more innovative administrative approach to infliximab treatment.
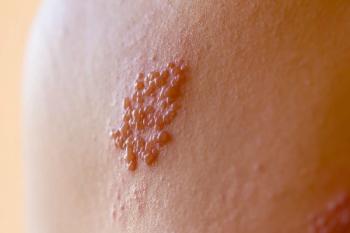
Patients with rheumatoid arthritis had a higher risk of shingles when treated once a day with 4 mg of baricitinib, 100 mg of peficitinib, or 30 mg of upadacitinib.

Despite pleas from celiac disease advocates, the FDA still does not require drug manufacturers to note on the label whether a medication contains gluten.

Anti-TNF agents were found to increase the risk of autoimmune disease, especially in psoriasis and lupus.
These medications join adalimumab-atto, which launched on January 31.

Quality of life (QOL) for people living with autoimmune diseases benefits from an integrated approach that considers individual immune system sensitivities and patients’ life circumstances, a new study shows.