
The drug was previously approved in 2009 to treat schizophrenia.

The drug was previously approved in 2009 to treat schizophrenia.

It can be hard to look away from a once in a lifetime total eclipse, but it’s important to understand the risks that come with viewing.

This year’s conference, which is being held April 4 to 6 in San Diego, California, features talks from industry experts on developments in the pharmacy field, actionable skills, and informative CE sessions.
Priovant Therapeutics said that it intends to initiate a phase 3 program of brepocitinib in the second half of this year.

Rejoyn from Otsuka Pharmaceutical and Click Therapeutics is a 6-week program that helps enhance cognitive control of emotions through brief therapy lessons and training exercises.

In comparison with the traditional volume-based pharmacy model, the membership model decreases financial barriers while increasing customer-pharmacist relationships.

The can’t-miss event on April 13 features speakers and CE sessions focused on elevating patient-centric care.

According to Anita Jacobson, PharmD, pharmacists should actively facilitate access to harm reduction programs by providing information and resources, ensuring the person who uses drugs doesn’t have to navigate the landscape on their own.
It’s important that pharmacists continuously revise their counseling as new information about substances such as xylazine, levamisole, and phenacetin emerges, said Anita Jacobson at this year’s American Pharmacists Association Annual Meeting & Exposition.

Check out a recap of important pharmacy news you might’ve missed this week, dispensed in small doses.

Researchers found various reasons for why individuals chose a specific pharmacy and how they purchase OTC medication.

Thanks to the Inflation Reduction Act, Medicare Part B coinsurance rates will lower if drug prices rise faster than the rate of inflation.
The approval of vadadustat (Vafseo) from Akebia Therapeutics was based on data from the phase 3 INNO2VATE program.
Following quality concerns voiced by the FDA regarding plastic syringes made in China, medical technology company BD has announced it will increase its production of plastic syringes in the US.

This week, a divided America watched as the Supreme Court heard oral arguments regarding whether to reinstate restrictions on accessing mifepristone, an essential drug used in medication abortion.

Tenofovir alafenamide was originally approved by the FDA in 2016 to treat adults with chronic HBV infection with compensated liver disease and in 2022 to treat pediatric patients aged 12 years and older.
At APhA 2024, 2 posters looked into how collaboration between physicians and community pharmacists is crucial when it comes to opioid prescriptions, and current opioid use patterns among older adults.

mRNA-1283 generated a stronger immune response against both the Omicron BA.4/BA.5 and the original virus strains of SARS-CoV-2 compared with Spikevax, Moderna’s licensed COVID-19 vaccine.

Two posters presented at APhA 2024 examined how the COVID-19 pandemic impacted pharmacists and their work.

Researchers detailed the FDA’s recommendations on low-dose aspirin and pharmacists’ role in cardiovascular practice transformation programs.
The drug had previously been granted a breakthrough therapy designation by the FDA.
This new drug utilizes the bactericidal properties of taurolidine and the bloodthinning effects of heparin to maintain catheter patency.

Posters presented at the APhA 2024 Annual Meeting and Exposition discussed pharmacist barriers in pediatric immunizations, a novel meningococcal vaccine, and the need for inpatient reviews of vaccination history.
In a poster presented at the 2024 American Pharmacists Association Annual Meeting and Exposition, researchers addressed continuous glucose monitoring and its potential role in indigent communities.

According to Sa’ed Al-Olimat, education and advocacy are key to ensure a safe and accessible landscape of psychedelic therapeutics, especially as the compounds inch closer to the mainstream health care market.
The Partnership for Safe Medicines found several product listings on Etsy that are designed to mimic regulated pharmaceuticals.

In a poster presented at the 2024 American Pharmacists Association Annual Meeting and Exposition, researchers assessed state-level regulations for PBMs.

Jeffrey Reese, president at Reese Pharmaceutical, discusses what sets ColoTest apart from other at-home colorectal cancer screening tests.
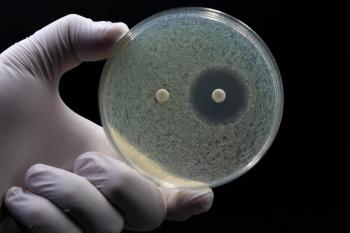
Researchers presented their findings on hypersensitivity caused by antibiotics as well as the best ways pharmacists can test for it.

In a conversation at this year’s American Pharmacists Association Annual Meeting & Exposition, Sa’ed Al-Olimat outlined the potential of psychedelic therapeutics in treating mental health disorders.