
Your weekly roundup of the latest news from Drug Topics®.
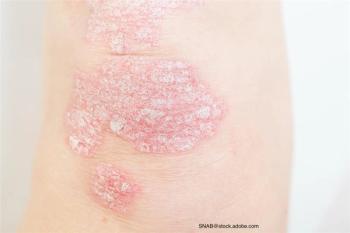
Nonsteroidal topical agents and TYK2 inhibitors may join an already full armamentarium.
Drug manufacturers are rejecting the program’s blanket discounts involving contract pharmacies. A flurry of litigation has ensued.

Consumers are purchasing dietary supplements that support or boost the immune system like never before.
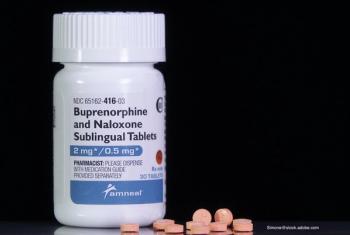
Opioid use disorder—how can we battle the epidemic of overdoses?

Your weekly roundup of the latest news from Drug Topics®.
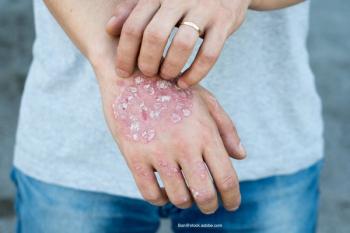
Examining the results of a study examining the role of supplements and diets in managing psoriasis.
Overall numbers may be lower, but the obese, racial/ethnic minorities, and those without health care access have higher numbers of undiagnosed diabetes

The rule change could save money for millions.

Patients are either skipping care completely, cutting costs elsewhere, or borrowing money to pay for health care
Oteseconazole is a treatment option for those living with a previously unmet medical need.

Your weekly roundup of the latest news from Drug Topics®.

More people getting health coverage, but 26.4 million still don’t have it.

A new CE opportunity that's available on-demand.

Arcutis Biotherapeutics’ roflumilast cream will provide relief to patients aged 12 years or older diagnosed with plaque psoriasis.

A joint statement from the two Democrats senators says prescription drug reform would save the federal government $288 billion over the next 10 years.

Your weekly roundup of the latest news from Drug Topics®.

Take a look back at our most popular news from July 2022.

New research adds more evidence against the supplement's efficacy in preventing fractures.
Third annual report from Optavise demonstrates why ongoing health benefits education is critical.

Pharmacogenomics looks at how genetic influences affect an individual’s response to therapeutic medications.

Your weekly roundup of the latest news from Drug Topics®.

The approval represents an adjunctive therapy option for adults and pediatric patients older than age 16.

Groups responded quickly in defense of pharmacists’ ability to prescribe Paxlovid through Test to Treat programs.

Your weekly roundup of the latest news from Drug Topics®.

The Big Three all set up group purchasing organizations recently, but some industry observers question the timing of the move and who will benefit.

Your weekly roundup of the latest news from Drug Topics®.

Several cases of liver injury have been identified in phase 3 clinical trials of tolebrutinib.

Your weekly roundup of the latest news from Drug Topics®.