The new approval is the first nonopioid option for moderate to severe acute pain in adults.

The new approval is the first nonopioid option for moderate to severe acute pain in adults.

This new drug utilizes the bactericidal properties of taurolidine and the bloodthinning effects of heparin to maintain catheter patency.
Anacaulase-bcdb improves current standard of care for deep partial thickness and/or full thickness thermal burns.

Approval followed resubmission of data from the phase 3 CONFIRM trial to the agency.
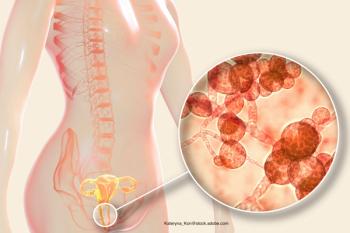
Oteseconazole is a treatment option for those living with a previously unmet medical need.
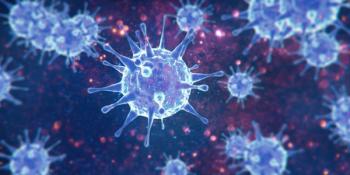
Molnupiravir is an oral antiviral for the treatment of mild to moderate COVID-19 in adults at high risk of severe disease.

Pfizer received an emergency use authorization (EUA) for Paxlovid, an oral pill for the treatment of mild to moderate COVID-19 in certain adult and pediatric populations.

On September 28, the FDA granted approval to oral atogepant (Qulipta) for the prevention of migraine.

Published: August 8th 2022 | Updated: