
The subgroup analysis shows adolescents treated with the drug once daily had higher rates of clear or almost clear skin compared to patients receiving the placebo.

The subgroup analysis shows adolescents treated with the drug once daily had higher rates of clear or almost clear skin compared to patients receiving the placebo.

Over 20 years since skin of color dermatology was introduced, researchers explored advancements and what is yet to come in the future.

The drug is approved for the short-term treatment of acute repetitive seizures, also known as seizure clusters, which differ from a patient’s normal seizure pattern.

At AAP 2025, Melanie Maxwell, president of AlignRx, discussed the intent, benefits and consequences of the IRA.

On behalf of 39 state and territory attorneys general, the National Association of Attorneys General issued a letter to Congress regarding major PBMs’ conflicts of interest.

Mark Garofoli, PharmD, MBA, BCGP, CPE, CTTS, discusses effective strategies for balancing excellent patient care with strict regulations surrounding controlled substances.

The FDA approved adalimumab-aaty in May 2023 for 8 indications, including rheumatoid arthritis, psoriatic arthritis, Crohn disease, ulcerative colitis, and plaque psoriasis.

One asymptomatic patient did experience drug-induced liver injury that was resolved after discontinuation of danuglipron.
At AAP 2025, Douglas Hoey, CEO of NCPA, discussed the possibility of PBM reform under the new administration and his take on Optum Rx’s plans to introduce a cost-plus reimbursement model.
In a telephone audit, researchers attempted to quantify the frequency of patient barriers when looking to purchase sterile syringes from community pharmacies for over-the-counter use.

First-in-class brensocatib has the potential to be the first approved treatment for bronchiectasis.

With transitions of care inclusive of multiple health care settings, each provider must play a specific role to ensure overall improved patient outcomes.

Researchers explored TikTok’s role in the dissemination of information regarding isotretinoin and its users.
At AAP 2025, Ron Friedman discussed best practices for responding to a DEA inspection, how to identify any red flags, and best practices for dispensing.
However, patients should still be monitored for potential serious adverse effects, as investigators identified potential new concerns.

Furthermore, C-reactive protein has greater prognosis for lung cancer, colorectal cancer, and ovarian cancer.

Allergy season is here. Help your patients tackle sneezing, itching, and congestion with this guide to the best OTC treatments.

DeLon Canterbury, PharmD, BCGP, discusses how his career journey led him to medication deprescribing and advocating for older adults.

Collaborative practice agreements enable pharmacists to leverage their expertise beyond traditional dispensing, but barriers continue to limit their implementation.
Researchers explored the association of a pneumococcal polysaccharide vaccine booster and health care utilization in children with sinusitis and low pneumococcal antibody titers.

The drug is also approved for adults with chronic inflammatory demyelinating polyneuropathy.
The new approval is the first nonopioid option for moderate to severe acute pain in adults.
The designation addresses the unmet need for prevention of H5N1, which remains a global health risk, and a phase 1 trial of the vaccine was initiated in November 2024.
Using a cost-utility analysis, researchers addressed the cost-effectiveness of pneumococcal vaccine strategies for adults over the age of 64.
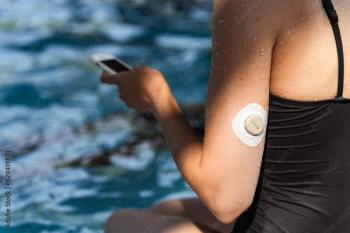
Pharmacists have positive impacts on health outcomes for diabetes and patients using CGM devices, including reductions in A1c.
Investigators find 5 distinct obesity trajectories that influenced brain morphology, function, and cognition.

Hypertension and obstructive sleep apnea are considered independent risk factors for cardiovascular diseases as well as diabetes.

Female pharmacists make up approximately 62% of the pharmacy workforce. They want, and in many cases demand, a good work-life balance.

In an update to the Journal of Pain Research’s formative meta-analysis on pain management in the US, researchers focused on the racial and ethnic disparities in access to pain treatment.

The drug shows a decrease in proteinuria and is well tolerated and safe, including for Black patients with chronic kidney disease.