
The drug will be made available in the second quarter of 2025.

The drug will be made available in the second quarter of 2025.

The approval of xanomeline and trospium chloride (Cobenfy) from Bristol Myers Squibb represents a “transformative moment in the treatment of schizophrenia.”

In the TEMPO-1 trial, both tavapadon doses effectively reduced motor symptoms in patients with early-stage Parkinson's disease.

Bezisterim acts on inflammatory signaling pathways and may be a viable treatment option for post–COVID-19 condition, or long COVID.

Results from HERCULES will form the basis for future discussions with global regulatory authorities, according to Sanofi.

In a clinical trial, IPX203 was able to achieve longer good on-time with less doses compared to standard treatment.
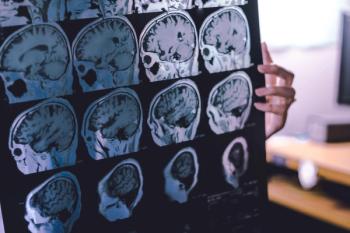
Liraglutide slowed the loss of volume in critical brain regions and cognitive decline in patients with mild Alzheimer's dementia.
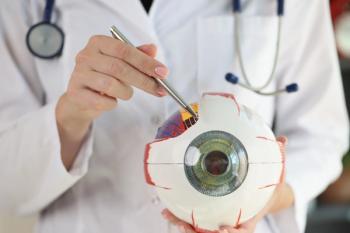
Researchers addressed the association between semaglutide use and the risk of nonarteritic anterior ischemic optic neuropathy.
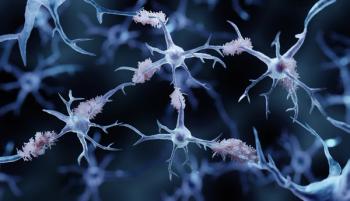
Donanemab-azbt was approved under the FDA's Fast Track Review, Priority Review, and Breakthrough Therapy designations.

For Migraine and Headache Awareness Month, Drug Topics spoke with Shivang Joshi, MD, MPH, RPh, FAHS to debunk migraine myths and explore resources to promote migraine awareness and education.

Shivang Joshi, MD, MPH, RPh, FAHS discusses how pharmacists can utilize their role as the most accessible health care professional to provide care to patients with migraine, as well as resources to enhance awareness and education.

Shivang Joshi, MD, MPH, RPh, FAHS, headache specialist neurologist at Community Neuroscience Services, debunks common migraine myths.
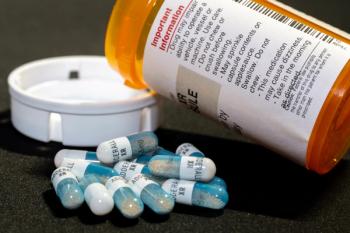
Stimulant medications are the first-line pharmacologic therapy for ADHD, but many of these medications are currently in short supply.

On January 6, 2023, the FDA granted lecanemab-irmb (Leqembi) accelerated approval for treating mild cognitive impairment and mild dementia in patients with Alzheimer disease.

After facing challenges, Biogen is discontinuing development and sales of aducanumab (Aduhelm) and focusing on lecanemab (Leqembi).