
A lack of clarity around certain provisions of the Inflation Reduction Act has presented challenges to payers.

A lack of clarity around certain provisions of the Inflation Reduction Act has presented challenges to payers.

A siloed approach to care can worsen patient outcomes, but collaboration between one health system specialty pharmacy and a manufacturer led to better patient care.

Understanding the goals of each party is the first step to a successful collaboration.
Collaboration between health system specialty pharmacists and pharmaceutical manufacturers can benefit patients in the long run.

Collaboration between health system specialty pharmacies and manufacturers can benefit patients in the long run.

Addressing requirements around FDA interchangeability designations can increase the adoption of biosimilar substitutions at the pharmacy level.

A recent bill introduced to Congress is intended to prevent product hopping, a practice that extends exclusivity and hinders the adoption of biosimilar therapies.

Distributors can help manufacturers create patient access programs that reduce costs, copays, and co-insurance.

One notable incentive includes the launch of biosimilars at both low- and high wholesale acquisition costs.

Pharmacists can help financial decision makers understand the value of biosimilar therapies.

Savings associated with biosimilar uptake are projected to reach $181 billion through 2027.

The role of health care specialty pharmacies has expanded.

The expertise of health system specialty pharmacists, combined with better adherence and better affordability, leads to better patient outcomes.

From medication adherence to patient counseling, clinical pharmacists at health system specialty pharmacies can play a role in reducing health care costs.

The clinical manifestations of shingles can be divided into 3 phases.

Despite a wealth of knowledge, pharmacists are frequently not consulted as part of the decision-making team in atrial fibrillation management.

This approval is the second biosimilar approval for manufacturer Alvotech.

Test your pharmacy news knowledge with our weekly quiz.
Fasenra was initially approved in 2017 as an add on maintenance therapy in patients aged 12 years and older.
Abrysvo is currently approved to treat RSV-associated lower respiratory tract disease in adults aged 60 years and older, and in infants through the immunization of pregnant individuals.
The antibiotic was approved to treat Staphylococcus aureas bacteremia, acute bacterial skin and skin structure infections, and community-acquired bacterial pneumonia.
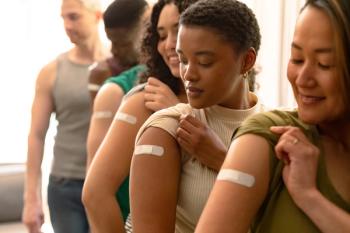
Understanding the role of pharmacies in vaccination rates can help prepare officials for the next public health emergency.

The drug was previously approved in 2009 to treat schizophrenia.

The agency issued warning letters to 6 companies manufacturing these products.
The drug had previously been granted a breakthrough therapy designation by the FDA.

The average US household spent $645 on OTC products in 2022.

Adults aged 65 years and older are the largest group of consumers of OTC medications.
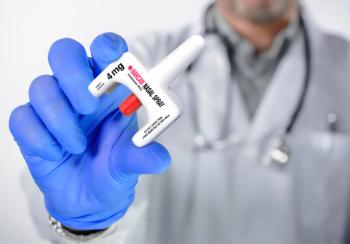
The first nonprescription, OTC naloxone nasal spray (Narcan) was approved by the FDA in March 2023.
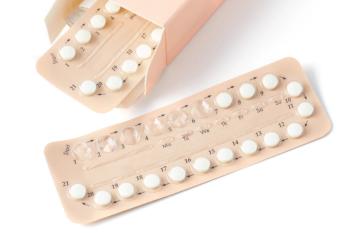
Access to counseling and education were strong predictors of being dispensed contraceptives.

These OTC products are recommended to address a variety of symptoms associated with women's health issues.