New Test Available for Varicella Zoster Virus Detection
ZosterGent Reagent, a fast fluorescent antibody test for the detection and confirmation of the varicella zoster virus, is now available for use with high-risk patients, including those who are immunocompromised.
ZosterGent Reagent, a fast fluorescent antibody test for the detection and confirmation of the varicella zoster virus (
The 1-step, 1-solution test delivers results in less than 30 minutes, according to cellular biologist and Viro CEO Nicholas Vafai, PhD, MBA. He founded the company with his father, microbiologist Abbas Vafai, PhD, a former chief of the biologics branch at the CDC and inventor of the shingles vaccine
“We think the Shingrix vaccine has been very effective, and we thought it was a great time to bring more attention to the actual diagnosis of shingles with a very simple, sensitive, and rapid test for the infection of the varicella zoster virus,” Dr Vafai told Drug Topics®.
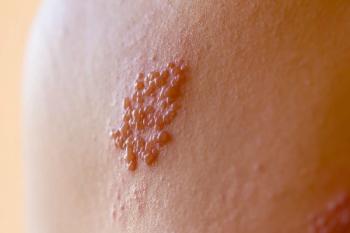
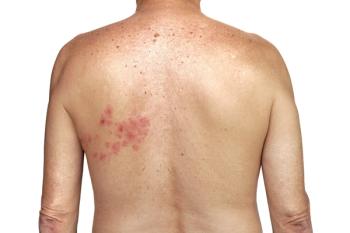
There are other tests for the detection of VZV—the virus that causes both chickenpox and shingles—such as PCR and ELISA. They can be processed in large batches, making them attractive to high-volume reference labs. "However, pathologists may be required to directly detect and observe the infected cells in skin lesions and in the patient's tissue under the microscope, which is why they would prefer ZosterGent,” Vafai noted.
ZosterGent is an immunofluorescent assay (IFA) approach that offers a mix of 6 highly unique and specific monoclonal antibodies. The process begins when a health care provider takes a sample from a patient’s lesion vesicles and sends it to a laboratory.
After preparing the sample, a lab technician views it under a fluorescent microscope. Positive samples appear with a bright green intensity that makes the specimen easy to see and recognize. The health care provider can receive test results within 24 hours.
“Immunofluorescent assays are one of the gold standards, highly specific and sensitive tests that have been used for decades now,” explained Vafai. “Research institutions and hospitals that have their own virology labs or diagnostic facilities onsite can know the result very quickly and be very confident that the result is accurate.”
Quick and accurate diagnosis of shingles is particularly important for ZosterGent’s target group, immunosuppressed individuals including patients with HIV or
Newsletter
Pharmacy practice is always changing. Stay ahead of the curve with the Drug Topics newsletter and get the latest drug information, industry trends, and patient care tips.