Bristol Myers Squibb announced the commercial US launch of ozanimod (Zeposia) for the treatment of relapsing forms of multiple sclerosis.

Bristol Myers Squibb announced the commercial US launch of ozanimod (Zeposia) for the treatment of relapsing forms of multiple sclerosis.
Impax requested that pharmacists who have dispensed Impax’s epinephrine injection, USP auto-injector 0.3 mg since December 20, 2018 contact all patients who received the device and provide instructions for inspection.

The study’s authors examined the opportunity to decrease the overall cost of bortezomib by $300-400 million by switching patients to the generic version.
Pharmacies and pharmacists can help the rapid deployment of COVID-19 vaccines when available, NACDS said.

IW-3718, an investigational adjunct therapy to proton pump inhibitors for refractory GERD, showed favorable results in reducing heartburn severity and other symptoms.
An analysis found that US patients with COVID-19 are experiencing longer hospital stays and higher rates of ICU admissions than patients in China.

Can coronavirus-related stress cause shingles?
This is the first regulatory approval for aspirin plus ticagrelor dual antiplatelet therapy for individuals with high cardiovascular risk but without prior instances of heart attack or stroke.
Officials with the FDA approved atezolizumab (Tecentriq, Genentech) in combination with bevacizumab (Avastin) for the treatment of patients with unresectable or metastatic hepatocellular carcinoma who have not received prior systemic therapy.

A systematic review of medical cannabis and cannabinoid use in oncology care demonstrated varied results.
Targeted therapy osimertinib (Tagrisso) demonstrated significant improvements in disease-free survival in patients with localized non-small cell lung cancer (NSCLC) who were treated following surgery.

Apotex Corp is voluntarily recalling all lots of metformin hydrochloride extended-release tablets, USP 500 mg, within expiry to retail level.

The study marks the first time that the drug has demonstrated benefit for front-line therapy in patients with this type of colorectal cancer.

An outpatient multiple myeloma clinic benefited substantially from employing a clinical pharmacist.
Here's this week's roundup of the latest coronavirus-related news.
“Data collection is ongoing with additional analyses planned to look at patient and provider perception of COVID-19 impact on cancer care.”
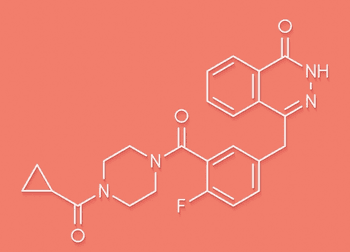
SOLO-2 is the first phase 3 trial to provide overall survival (OS) data on maintenance PARP inhibitor therapy.

The study is the first of its kind on a national scale to show the benefit of Medicaid expansion on cancer mortality rates.
CMS is reducing insulin copays for seniors who are eligible for Medicare via an executive order from President Donald Trump.

The findings, which were presented virtually in the 2020 ASCO Virtual Scientific Program, included the association between treatment with hydroxychloroquine plus azithromycin and increased risk of death.

Months after FDA said it was investigating the carcinogenic ingredient NDMA in metformin products, the agency’s testing has found elevated levels of NDMA in extended release metformin, but not in the immediate release formulation.

The American Society of Clinical Oncology (ASCO) recently released a detailed guidance to oncology practices

In a new consensus paper, experts emphasized that high doses of vitamin D supplementation have no current benefit in the prevention or treatment of COVID-19.
Although several therapeutic agents have been evaluated for the treatment of COVID-19, none have yet shown to be effective.
Results from a phase 3 study showed that galcanezumab-gnlm (Emgality, Eli Lilly) significantly improved work productivity and health and well-being between migraine attacks.

Officials with the FDA have granted priority review for mepolizumab (Nucala, GlaxoSmithKline) in the treatment of patients with hypereosinophilic syndrome.
Filgotinib achieved all primary end points of the phase 2b/3 study in patients with moderate-to-severe ulcerative colitis.

Dupilumab (Dupixent, Sanofi and Regeneron) is the first biologic medicine approved for treatment of moderate-to-severe atopic dermatitis.

In an interview with Drug Topics®, Richard Scholz, chief strategy officer of ERxDirect, explained the prominence of telemedicine during the COVID-19 pandemic and the lasting implications of new technologies during unprecedented times.

New data showed that treatment with hydroxychloroquine was associated with increased mortality in hospitalized patients with COVID-19.