The updated analyses of the AZD1222 COVID-19 vaccine will be submitted to the FDA for emergency use authorization, according to AstraZeneca.

The updated analyses of the AZD1222 COVID-19 vaccine will be submitted to the FDA for emergency use authorization, according to AstraZeneca.

AstraZeneca is preparing to submit its COVID-19 vaccine data to the FDA for emergency use authorization in the coming weeks.
The CDC reported data demonstrating high adherence to COVID-19 vaccine doses among recipients.

Here’s a preview of the featured content to watch for this week on Drug Topics®.
Here’s a roundup of the latest coronavirus-related news.

NCPA lauded the higher reimbursement rate in a statement.

Here’s a preview of the featured content to watch for this week on Drug Topics®.

Here’s a roundup of the latest coronavirus-related news.

Total Pharmacy® will run as an insert in the Drug Topics® publication and host content in an additional online environment.

Company launches new Global Sustainability microsite and ESG Reporting Index .

Here's a roundup of the latest coronavirus-related news.
Results of a new national survey showed that more than 8 in 10 adults prefer their local pharmacists over mail order services.

Former high touch technologies exec to bring customer service experience, expertise.
Here’s a preview of the featured content to watch for this week on Drug Topics®.

The FDA is allowing more flexible storage and transportation conditions for Pfizer-BioNTech’s COVID-19 vaccine.

The FDA has granted emergency use authorization status for the first single-dose vaccine for COVID-19 in the United States.
Here’s a roundup of the latest coronavirus-related news.

On Friday, an advisory panel will meet to evaluate the vaccine candidate and decide whether to recommend for emergency use authorization.

Here’s a preview of the featured content to watch for this week on Drug Topics®.

Members of the MJH Life Sciences™ COVID-19 Coalition recently weighed in on the latest coronavirus disease 2019 (COVID-19) pandemic news.

Here’s a roundup of the latest coronavirus-related news.

PrimeRx™ pharmacies can utilize the interface to facilitate the registration, recordkeeping and reporting requirements for the administration of all vaccinations, including COVID-19 vaccines.

The MJH Life Sciences™ COVID-19 Coalition is hosting a live webinar event on the legal and ethical considerations of COVID-19 vaccination.
The US government has purchased 100 million more vaccine doses for COVID-19 to stay ahead of increasing demand.

Here’s a preview of the featured content to watch for this week on Drug Topics®.

Former McKesson exec to lead Keycentrix technology development.

Here's the roundup of the latest coronavirus-related news.

Alliance will advance and promote career opportunities for pharmacists, pharmacy technicians, and other key pharmacy staff across the country
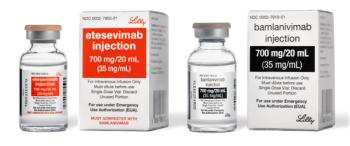
The emergency use authorization, which was issued to Eli Lilly, grants the use of bamlanivimab in combination with etesevimab for mild-to-moderate COVID-19 infection in adult and pediatric patients.