
Here’s a roundup of the latest coronavirus-related news.

PrimeRx™ pharmacies can utilize the interface to facilitate the registration, recordkeeping and reporting requirements for the administration of all vaccinations, including COVID-19 vaccines.

The MJH Life Sciences™ COVID-19 Coalition is hosting a live webinar event on the legal and ethical considerations of COVID-19 vaccination.
The US government has purchased 100 million more vaccine doses for COVID-19 to stay ahead of increasing demand.

Here’s a preview of the featured content to watch for this week on Drug Topics®.

Former McKesson exec to lead Keycentrix technology development.

Here's the roundup of the latest coronavirus-related news.

Alliance will advance and promote career opportunities for pharmacists, pharmacy technicians, and other key pharmacy staff across the country
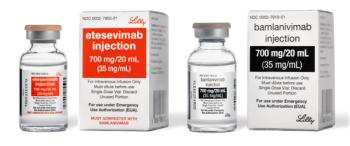
The emergency use authorization, which was issued to Eli Lilly, grants the use of bamlanivimab in combination with etesevimab for mild-to-moderate COVID-19 infection in adult and pediatric patients.

Here’s a preview of the featured content to watch for this week on Drug Topics®.
The FDA advisory committee will meet in 3 weeks to discuss the Janssen investigational vaccine.
Here's the roundup of the latest coronavirus-related news.
Starting February 11, federal vaccine supplies will be delivered directly to select pharmacies, according to the announcement.

Here’s a preview of the featured content to watch for this week on Drug Topics®.

Here’s a roundup of the latest coronavirus-related news.
The Biden administration announced efforts to boost COVID-19 vaccination supply and increase transparency.

Here’s a preview of the featured content to watch for this week on Drug Topics®.

Here's the roundup of the latest coronavirus-related news.

Alliance will provide insight, education, and training opportunities to pharmaceutical and life sciences professionals.

In its next live webinar event, MJH Life Sciences™ COVID-19 Coalition will explore emerging mutations of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).

Apart from COVID-19, there are several other industry trends taking root that will likely have a transformative effect on the profession in years to come.

Users of the PrimeRx pharmacy management technology system now have direct integration access to Surescripts Real-Time Prescription Benefit.

Here’s a preview of the featured content to watch for this week on Drug Topics®.

President-elect Joe Biden revealed his COVID-19 plan, which includes a disciplined focus on vaccination efforts.
Here's the latest coronavirus-related news.

In a final briefing, OWS recommended states expand vaccination to all individuals 65 and older or those with comorbidities.

Here’s a preview of the featured content to watch for this week on Drug Topics®.
The FDA has identified 3 molecular tests that may be impacted by an emerging SARS-CoV-2 variant.

AmerisourceBergen has announced its acquisition of the majority of Walgreens Boots Alliance’s Alliance Healthcare businesses.

In a statement, FDA officials recommended that all health care professionals continue to adhere to the FDA-authorized dosing and schedules of the COVID-19 vaccines.