The CDC confirmed another human bird flu case, marking the first instance of the virus in a human without occupational exposure to infected birds or dairy cattle during the 2024 outbreak.

The CDC confirmed another human bird flu case, marking the first instance of the virus in a human without occupational exposure to infected birds or dairy cattle during the 2024 outbreak.
Whereby gastroparesis can hinder the absorption of oral medications in the stomach, Tonix Pharmaceuticals is promoting alternative migraine treatments that bypass the digestive system.

LEO Pharma presented data from 2 of the largest multinational real-world studies on the prevalence, severity and treatment of chronic hand eczema at the European Society of Contact Dermatitis congress.

Lingo offers users a real-time glucose graph, a metric to track glucose spikes, food and activity logging, personal recommendations, and challenges to motivate healthy behaviors.

Researchers analyzed a co-developed pharmacy-based intervention for individuals who have experienced domestic abuse or suicidal ideation.

Nymalize is an effective alternative to capsule extraction for adult patients with subarachnoid hemorrhage who are unable to swallow capsules.

Check out important updates from the FDA for the week of September 2.

As Medicare open enrollment begins, the NCPA is urging CMS to facilitate appropriate PBM contracting for pharmacies in the upcoming plan year.
Findings were presented at the American Heart Association's Hypertension Scientific Sessions in Chicago, Illinois.
CRS3123 is a novel, small molecule, antibiotic drug candidate that selectively inhibits 1 form of the bacterial methionyl-tRNA synthetase enzyme and is not affected by resistance to any existing classes of antibiotics.

McKesson, Cardinal Health, and Cencora will share the settlement payment, resolving claims that the companies were significantly involved in fueling the US opioid epidemic.

DaylightRX from Big Health is a digital formulation of cognitive behavioral therapy and is available by prescription only.

Embecta’s new patch pump offers a larger insulin reservoir to better accommodate for the needs of patients with type 2 diabetes (T2D).

Click Therapeutics announced that CT-132 met its primary endpoint in the phase 3 ReMMi-D trial.

Dana Krug, Senior Vice President of Cold Chain Fulfillment at Phononic, explained the current state of his company’s cooling technology and the common trends of temperature control throughout the industry.

Results from the PALISADE study were published in The New England Journal of Medicine.
The vaccine, VAX-31, targets 20 serotypes in common with PCV20, as well as 11 additional serotypes.

Results from HERCULES will form the basis for future discussions with global regulatory authorities, according to Sanofi.
The protein-based vaccine includes a monovalent component corresponding to the Omicron variant JN.1 strain.
Catch up on important immunization news from the month of August.
In case you missed it, this week we had news about COVID-19 and the upcoming respiratory virus season, advancements in clinical trials for ASCVD, the first OTC birth control, and more.
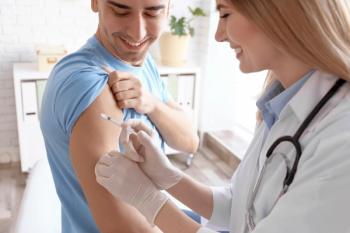
Chijioke Bennett, executive director of clinical development at Novavax, discusses how health care providers can increase awareness around the importance of COVID-19 vaccines.
Emergent BioSolutions has announced that it will donate 50,000 doses of ACAM2000 for potential deployment across affected areas in Central Africa.
Catch up on important diabetes news from the month of August.

Checkout these important FDA updates from the month of August 2024.
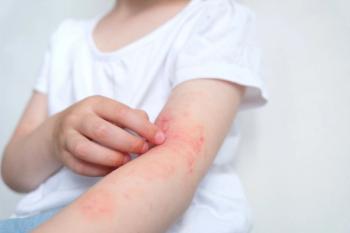
Results from the INTEGUMENT-OLE phase 3 trial showed the therapy was also well-tolerated, with no new safety signals observed during treatment of up to 56 weeks.
Stephanie Sober, MD, MSHP, investigator on the pivotal research that supported the prescription-to-OTC switch of Opill, shares insights on how the medicine is reshaping the reproductive landscape.
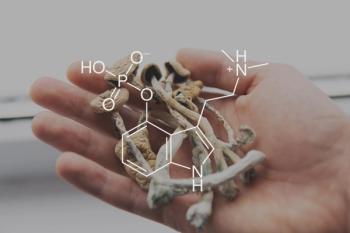
As research continues to uncover the potential benefits of psilocybin, pharmacists and other clinicians have an essential role in bridging ancient wisdom with contemporary science.

Tris Pharma will examine the efficacy and safety of cebranopadol in the ALLEVIATE-1 and ALLEVIATE-2 studies.

Check out this list of our top 10 stories from August 2024.