
The vaccine mandate applies to employees at facilities that participate in the Medicare and Medicaid insurance programs.

The vaccine mandate applies to employees at facilities that participate in the Medicare and Medicaid insurance programs.

After a panel federal judges put a temporary halt on the mandate, the U.S. Surgeon General says the administration is ready to fight for the requirement.
Pharmacists in 2 states are now empowered to administer FDA approved, CDC recommended vaccines.

In an exclusive interview with Drug Topics, Beth Mitchell, senior director of government affairs at AmerisourceBergen, explores the ways that pharmacists can take action on legislation that affects their practice.

Selling CBD can be profitable for pharmacies, if they follow regulatory guidelines.

AmerisourceBergen and Good Neighbor Pharmacy are working to support policies that will help advance the pharmacy profession.
President Biden issued an executive order, with a plan unveiled today pushing the US Department of Health and Human Services to increase support for biosimilars to combat high prescription drug prices.
PBMs are fighting to keep patients and consumers in the dark on prescription drug costs and attempting to reverse a rule created to promote drug price transparency.

WSPA, NPCA, and NACDS fully support the court decision requiring CMS to reconsider the below-cost reimbursement plan.

Here’s a roundup of the latest news coverage from Drug Topics®.

This special Over the Counter episode, brought to you by Total Pharmacy® and Omega Pharmacy Group, dives into how to navigate the business of independent pharmacy and get ahead of key policy changes.

The legislation would reduce patients’ cost sharing, prevent plan and PBM clawbacks, enhance price transparency, and establish clear performance measures for pharmacies.
Vaccination activities build on collective success of COVID-19 response over the past year

Bill S.1388 would require the FTC to examine recent merger activity of PBMs and how it may be affecting patients and independent community pharmacies.
In the final episode of the series, Dr. Lee highlights what's next in enhancing patient care for those with limited English proficiency.
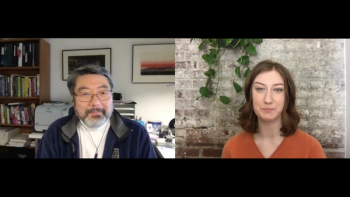
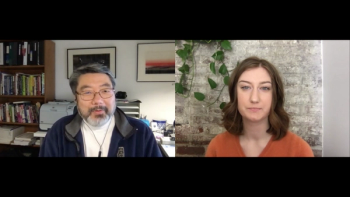
In part 2 of our interview, Dr. Charles Lee of FDB dives into the current challenges in medication errors for patients with limited English proficiency, his personal relation to this issue as a first-generation Korean immigrant, and where other states are at in providing patients with translated prescriptions.

In the first part of our interview, Dr. Charles Lee will explain what this new Oregon law stipulates, the significance of this legislation, and some of the consequences of medication errors due to patient language barriers.

NCPA applauded the Michigan’s House passage of the legislation, which would reform many PBM reimbursement practices in the state.

In part 1 of our podcast series with American Associated Pharmacies (AAP), AAP President and CEO Jon Copeland dives into key pharmacy industry trends.

NCPA lauded the higher reimbursement rate in a statement.

An update on COVID-19 advocacy activity and regulations affecting your pharmacy practice.

Pharmacy leaders have leveraged their experience, their role in interdisciplinary teams, and technology to quickly reinvent approaches to manage long-standing issues exacerbated by the pandemic.

The MJH Life Sciences™ COVID-19 Coalition is hosting a live webinar event on the legal and ethical considerations of COVID-19 vaccination.

SCOTUS' decision upholds an Arkansas law regulating PBMs’ drug reimbursement rates.

The Department of Health and Human Services recently released a final rule requiring health insurers to disclose drug pricing and cost-sharing information

Following the 2020 election, pharmacy groups welcomed the newly elected pharmacist congressmembers and commented on pharmacy issues requiring attention from Congress.

Guest host Brian Nightengale, president of Good Neighbor Pharmacy, is joined by Rich Tremonte and Doug Hoey to discuss the biggest opportunities and headwinds facing independent pharmacies in 2021.

Todd Mizeski, Esq, a Partner in Frier Levitt’s Life Sciences, Litigation Department, discusses the case of Rutledge v. Pharmaceutical Care Management Association.

Walmart filed a pre-emptive suit against 2 government agencies that are threatening to sue Walmart over its pharmacists filling opioid prescriptions.