
Cannabidiol (CBD)
Latest News
Latest Videos
CME Content
More News

The companies are marketing products that may violate Federal Trade Commission prohibition of “unfair and deceptive acts in the marketplace.”

Four abstracts presented at ASCO 2024 delved into cannabis and pain management, potential medication interactions, and symptom mitigation, in patients with cancer.

Researchers addressed the use of cannabis to treat cancer symptoms in rural and urban areas, as well as patients’ comfortability discussing it with their providers.



The agency intends to work with Congress to create a pathway that balances the desire for access with regulatory oversight and risk management.

Findings from a study in JAMA Network Open reveal valuable insight into the way cannabis legalization has impacted the perceptions of pregnant women who use cannabis in the US.

In the midst of an opioid epidemic, CBD may help patients experiencing opioid withdrawal symptoms find relief.

CBD is a hot topic on the internet and social media, but large percentages of posts are making therapeutic claims that are not FDA approved or backed by enough research.

Research examines how cannabidiol impacts plasma endocannabinoid when consumed along with delta-9-tetrahydrocannabinol.

With the rise of cannabidiol (CBD) use, understanding how it can impact the effect of other medications is important.
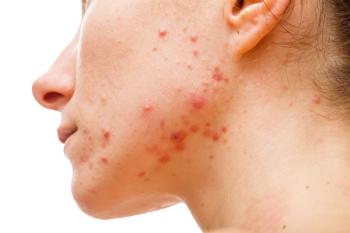
CBD therapy has had positive outcomes in other inflammatory conditions.

Cannabinoids lack the habit-forming mechanism of action found in methadone and buprenorphine, unlocking new potential for the drug.

Drug Topics® is joined by Dr. Alex Capano, Chief Science Officer at Ananda Professional to discuss what CBD is used for mental health, where research should go next, and how to ensure that patients are getting effective CBD products.

Drug Topics® is joined by Dr. Alex Capano, Chief Science Officer at Ananda Professional to discuss what CBD is used for in dermatology.

No federal agency has taken charge of regulating cannabidiol products, leaving decisions up to the individual states.

CBD can be used to treat a myriad of women’s health conditions, but research is still limited.

Tips to help your pharmacy stand out as a knowledgeable CBD retailer.

Encouraging outcomes in recent studies have researchers calling for more analysis of the effect of cannabinoids on sleep disorders.

Addressing patient concerns about cannabidiol can be the key to patient satisfaction.
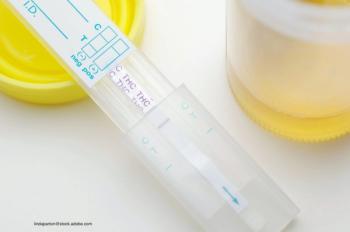
Even “THC-free” cannabidiol products may contain trace amounts of THC.

CBD products range from gummies to tinctures to topical lotions and creams.

CBD is still a gray area for many pharmacists and health care providers.

Patients should start low, go slow, and track their own dosing and effects

Keeping OTC products near the pharmacy counter creates an opportunity for patient counseling and education.


























